INTRODUCTION
The family Cystocloniaceae Kützing (Order Gigartinales F. Schmitz) includes ca. 100 species, in 13 genera that are characterized by uniaxial, terete or flat thalli, filamentous or pseudoparenchmatous medulla, zonately divided tetrasporangia, and procarps with 3- or 4-celled carpogonial branches (Womersley 1994, Saunders et al. 2004, Guiry and Guiry 2017). Hypnea J. V. Lamouroux, the most speciose genus in the family with 62 species, generally has terete thalli that are erect or entangled (Guiry and Guiry 2017), but their male and female structures are inadequately understood (Kylin 1930, Mshigeni 1976, Vázquez-Delfín et al. 2016). Recent molecular studies have confirmed Hypnea as a strongly supported monophyletic group that is closely related to the genus Calliblepharis Kützing (Saunders et al. 2004, Geraldino et al. 2010).
Although the inclusion of molecular data has become commonplace in modern taxonomy (Yang et al. 2015, Calderon and Boo 2016, Won et al. 2016, Yang and Kim 2016), reproductive structures have remained important diagnostic characteristics for differentiating many genera in the order Gigartinales (Hommersand et al. 1999, Díaz-Tapia et al. 2013). For instance, Hypnea and Calliblepharis can be readily distinguished each other by female reproductive structures even if they share many vegetative features in common. Although carposporangia are formed as terminal cells of each gonimoblast filament in both Hypnea and Calliblepharis, each gonimoblast filament produce only one carposporangium in Hypnea, while Calliblepharis exhibits in chains. Also, numerous filaments link the gonimoblasts and the pericarp in Hypnea, but these filaments are absent in Calliblepharis (Kylin 1928, 1930, 1932).
Hypnea saidana Holmes is a red algae characterized by complanate and more or less dichotomous thalli with alternate branches that are mostly curved bearing short branchlets (Holmes 1895, Okamura 1909, Tanaka 1941). First described based on the material collected from Enoshima, Japan (Holmes 1895), it has remained one of the most taxonomically well characterized species in Korea and Japan. However, recent development of phylogenetic methodology made the need for improving H. saidana taxonomy based on obsolete evidence urgent. For example, current classification of H. saidana lack the consideration of reproductive structure, according to Shin and Boo (1994) which is essential for proper reassessment due to its similarity to Calliblepharis in terms of vegetative features, i.e., complanate thalli, dispersed surface cells, and numerous pit connections between medullary cells. In addition, recently described taxa, Calliblepharis hypneoides Díaz-Tapia, Bárbara & Hommersand and C. psammophilus D’Archino & W. A. Nelson, were assigned to Calliblepharis based on observations of the female structures even though they superficially resembled the species of Hypnea (Díaz-Tapia et al. 2013, D’Archino et al. 2015).
However, taxonomy based on the reproductive characteristics, though effective, poses a serious problem in identifying appropriate specimens during the asexual stage of red algae (Sherwood et al. 2010) and molecular procedures have been used to circumvent this problem. In fact, these molecular procedures have rapidly redefined phylogenetic relationships among Gigartinales taxa recently (Yang et al. 2015, Calderon and Boo 2016, Yang and Kim 2016). Several molecular studies have also been performed to verify the phylogenetic relationships of species within genus Hypnea itself (Geraldino et al. 2009, 2010, D’Archino et al. 2015).
These considerations clearly reveal the possibility that current taxonomy of H. saidana based on vegetative morphology could be mistaken as Díaz-Tapia et al. (2013) mentioned, although some red algal species can be assigned to Hypnea based on morphology, they could be assigned equally well to Calliblepharis. This implies that phylogeny regarding species of these two genera requires highly refined morphological characterization and molecular analysis using diverse species enough to allow inter-genera comparison within the order Gigartinales.
In this study, we tried to reexamine H. saidana phylogeny (Okamura 1909, Tanaka 1941, Shin and Boo 1994, Yamagishi and Masuda 1997) to put it on a more accurate taxonomic position. As mentioned above, the family Cystocloniaceae requires the concentrated study using both molecular and morphological data to infer their phylogenetic relationships. Therefore, we put special emphasis on precise morphological characterization of reproductive structures, which was possible through direct observation of female and male reproductive structures of wild specimens. We also noted that our morphological characterization tightly supported our molecular analyses using the combined alignments of the nuclear-encoded small subunit ribosomal DNA (SSU) and chloroplast-encoded large subunit of the RuBisCO gene (rbcL) sequences.
MATERIALS AND METHODS
Specimen preparation
Samples of Calliblepharis saidana were collected in Korea and Japan during 2012–2016 (Table 1). Vegetative specimens were collected year-round, but female/male specimens were sampled only in the Jeju (August 2015) and Wando (January 2016), Korea. Field collections were kept fresh in a cool box containing seawater and transported to the laboratory. Voucher specimens were deposited to the Herbarium of Jeju National University and the National Institute of Biological Resources (NIBRRD0000000005), Incheon, Korea. Sub-samples were dried in silica gel for molecular analyses or in 5% formalin/seawater for morphological observations. Sections were hand cut using a razor blade or prepared using a bench-top freezing microtome (MFS No. 222; Nippon Optical Works, Tokyo, Japan). Section samples were stained with 1% aniline blue, acidified with 1% HCl and mounted in a 40% corn syrup solution. We also treated with Wittmann’s (1965) aceto-iron-hematoxylin-chloral hydrate, and mounted in 50% Hoyer’s mounting medium (Lin et al. 2009). Photomicrographs were captured using the EOS 600 D digital camera (Canon, Tokyo, Japan) mounted on the BX 43 microscope (Olympus, Tokyo, Japan). Images of pressed specimens were captured using the D 80 camera (Nikon, Tokyo, Japan). The digitized images were imported into Adobe Photoshop (ver. 6.1) software and edited to produce the photographic plates.
DNA extraction and sequencing
DNA was extracted from small portions of dried thalli in liquid nitrogen using the DNeasy Plant Mini kit (Qiagen, Hilden, Germany) following manufacturer’s instructions. The extracted DNA was stored at −20°C, and used to amplify chloroplast rbcL and nuclear SSU rDNA. The primer combination F7-R898 and R762-R1442 was used for rbcL (Gavio and Fredericq 2002, Kim et al. 2010) and G01–G14 and G04–G07 for SSU rDNA (Saunders and Moore 2013), respectively. Polymerase chain reaction (PCR) amplification was performed in the Swift MaxPro thermal cycler (ESCO, Singapore) using AccuPower PCR Premix (Bioneer, Daejeon, Korea). PCR products were purified using the Accuprep PCR Purification Kit (Bioneer) following manufacturer’s instructions. Sequencing of the forward and reverse strands of the purified PCR products was performed commercially by Macrogen (Seoul, Korea). Two electropherogram outputs from each sample were processed using Chromas version 1.45 software (Technelysium Pty Ltd., South Brisbane, QLD, Australia).
Phylogenetic analyses
Sequences generated in this study and sequences obtained from GenBank were aligned using Clustal O software (Sievers et al. 2011), and adjusted visually. We used sequences of 24 Gigartinales taxa for SSU and 41 Gigartinales taxa for rbcL, respectively. The sequence alignment of rbcL (a protein-encoding gene) was unequivocal due to the absence of indels while ambiguous sites of SSU rDNA were trimmed for the alignment. The SSU and rbcL alignments were concentrated and a combined data set were analyzed using maximum likelihood (ML) and Bayesian inference (BI) mentioned the following methods.
To infer phylogenetic relationships, we conducted ML analysis using the GTR + Γ evolutionary model (‘-m GTRGAMMA’ option) in RAxML (Stamatakis 2006). To identify the best-fit tree, we performed 200 independent tree searches using ‘the number of runs’ option. We ran 1,000 replications in RAxML software using the same setting to generate bootstrap support value of monophyletic nodes (MLBt). We also performed the BI in MrBayes v.3.2.1 (Ronquist et al. 2012) using the metropolis-coupled Markov Chain Monte Carlo simulations with the GTR + Γ model to select the most appropriate phylogenetic tree. For two independent runs, we computed 2 × 106 generations with four chains, and sampled the trees every 100 generations. The burn-in point was identified graphically by tracking the likelihoods when the plateaued. Eventually, total 29,000 trees sampled in the stationary state were used to infer Bayesian posterior probabilities (BPP).
RESULTS
Morphology of Calliblepharis saidana
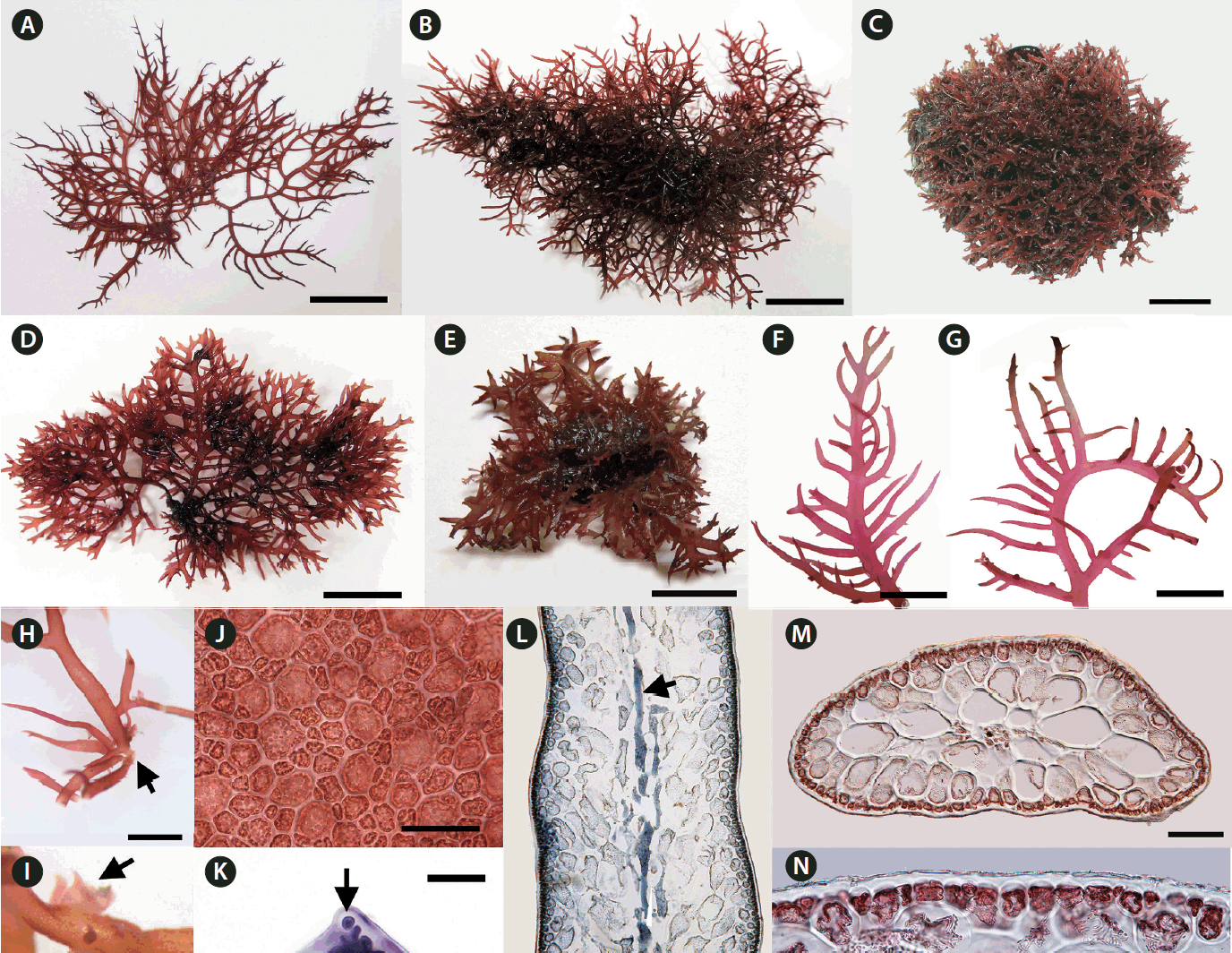
We collected Calliblepharis saidana from lower intertidal to subtidal zones where plants were attached to small pebbles, shellfish, other seaweeds, or drifted near the shoreline. Thalli of C. saidana were erect, compressed, 2–8 cm tall, 0.5–2.0 mm wide, and 400–850 μm thick, formed cartilaginous rounded clumps (Fig. 1A–E, L & M). Branches appeared dichotomous or alternate of erect thalli or unilaterally in outward side of secund thalli. They were usually curved, tapered to points, and bore short branchlets. Branching took place most often in a single plane but sometimes in three dimensional space (Fig. 1F & G). Holdfasts were discoid and multiple thalli arose from a single holdfast (Fig. 1H). Small projections attached to the substratum or adjacent branches were observed in thalli and branches (Fig. 1I).
On a surface view, cortical cells of a thallus were of various convex polygonal shapes and they did not form distinct rosettes (Fig. 1J). A thallus grew uniaxially initiated by a spearheading apical cell (Fig. 1K). It progressed through oblique divisions producing newly differentiated apical cells in alternating sides of growth axis. Longitudinal sections of the mid-frond (Fig. 1L) showed an axial filament, the trace of apical cell strand during growth, surrounded by elongated cells linked through secondary pit-connections. In the cross-sectional view, the central core of axial filament and periaxial cells were surrounded by two to four layers of large, rounded medullary cells measured 90–280 × 85–160 μm (Fig. 1M). The cortex consisted of a layer of rounded or cuboidal cells with 10–30 μm in diameter (Fig. 1N).
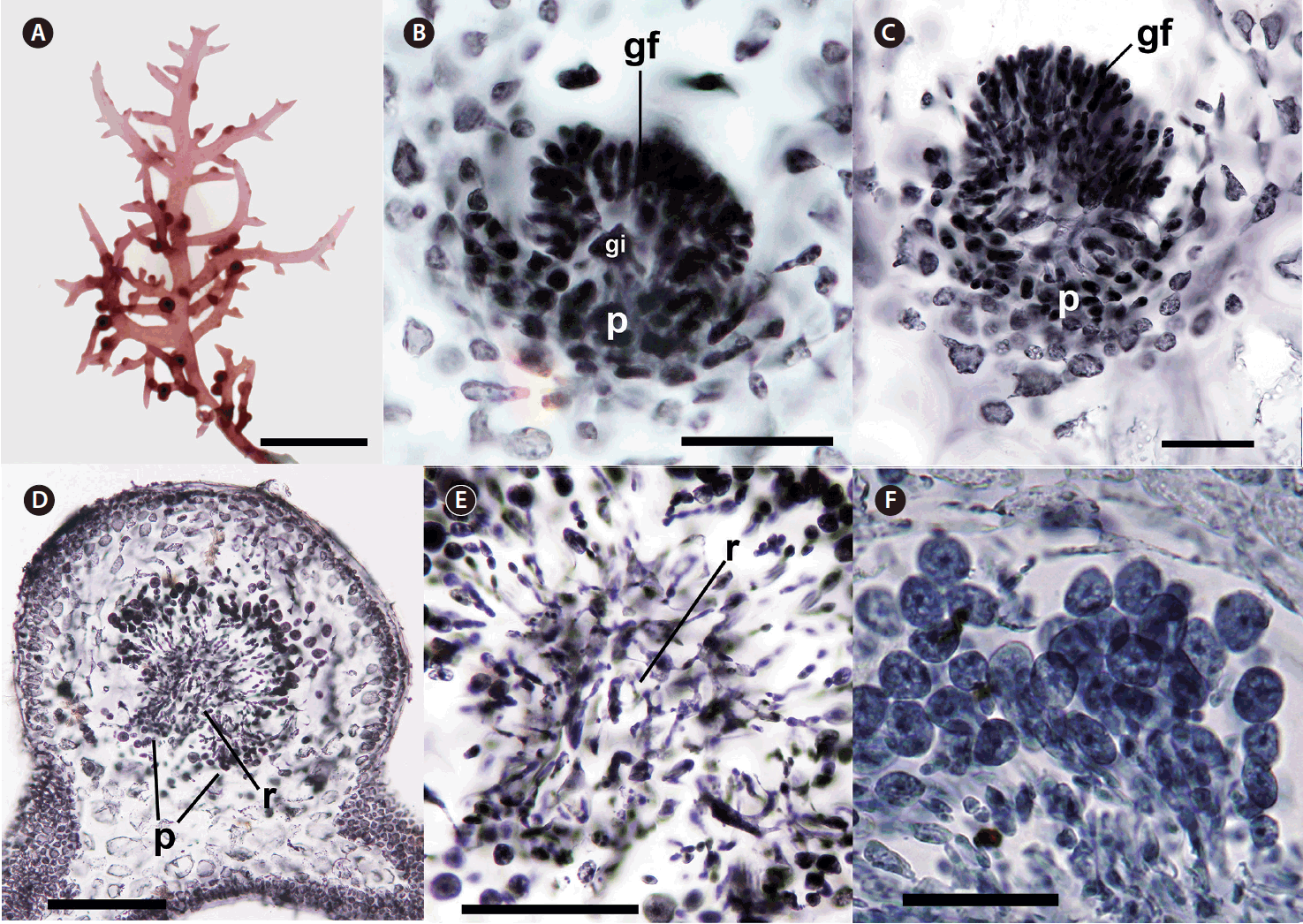
Cystocarps with gonimoblast initial, gonimoblast filaments, and placenta were formed inside branches and branchlets first and protruded through the cortex near their bases or sprouting points during maturation (Fig. 2A–D). When fully matured, they were sessile, subglobose with 600–800 μm in diameter encased in a thick pericarp without any ostiole (Fig. 2D). Nutritive placentae were generated at inward position from gonimoblast initial (Fig. 2B). Gonimoblast filaments were developed outwardly from gonimoblast initial and progressively turned into carposporangial chains (Fig. 2B–D). Small, interconnected cells between gonimoblasts and placenta formed a reticulum (Fig. 2D & E). Mature globose carposporangia of 15–30 μm diameter piled up just outside the gonimoblast reticulum (Fig. 2F).
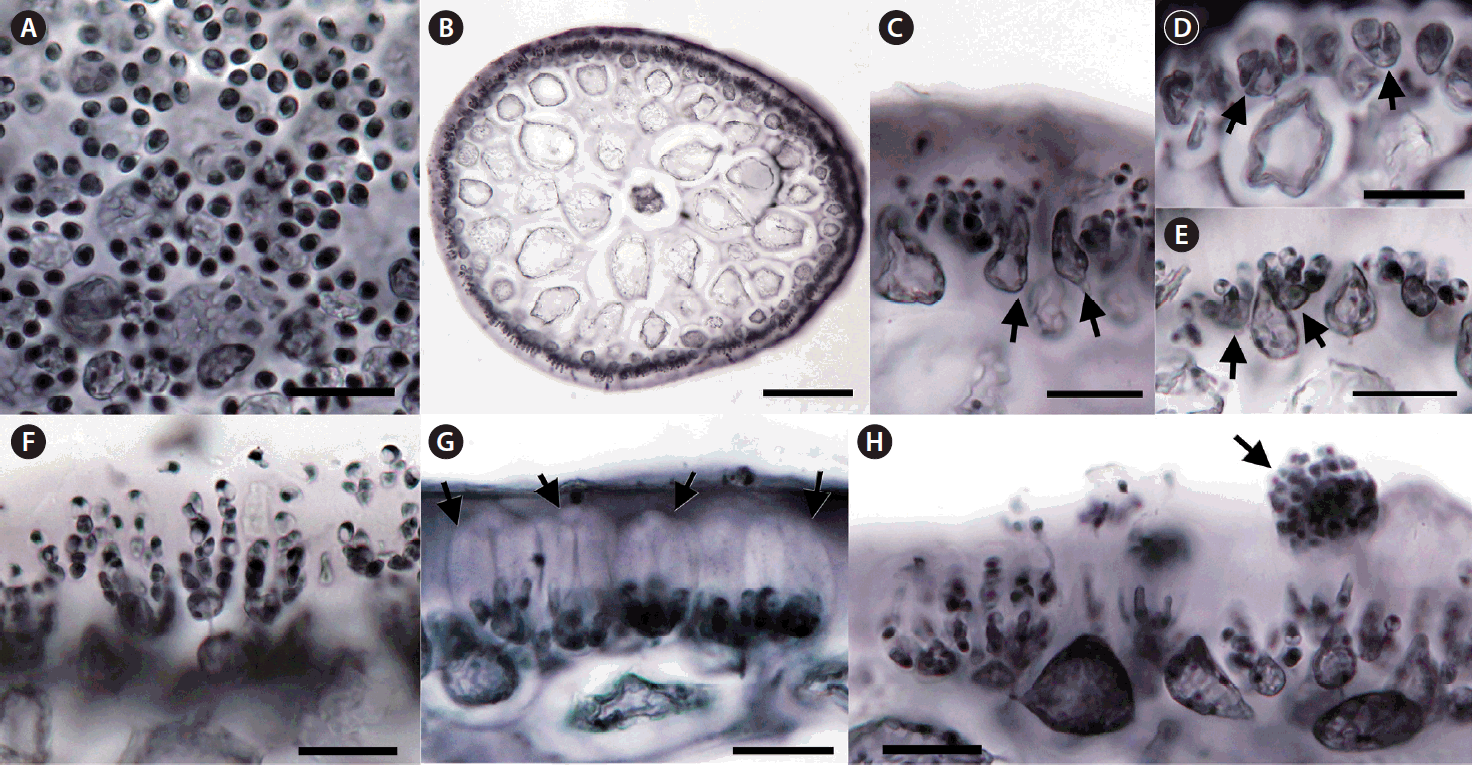
The spermatangia formed sori on branches and branchlets. Surface views of male plants showed rounded spermatia with 3–5 μm in diameter (Fig. 3A). A gelatinous matrix covered the reproductive portion of the thallus (Fig. 3B). Some of cortical cells became elongated (Fig. 3C), formed spermatangial mother cells (Fig. 3D), and produced spermatia (Fig. 3D–F). Spermatia existed in chains stacked up to seven cells (Fig. 3F). The mature antheridium was enclosed in a thick wall cushioned by an interposed gelatinous layer (Fig. 3G). Matured spermatia broke free from spermatangial chains, aggregated at the apex of the wall, and released through an apical split in the antheridial wall (Fig. 3G & H).
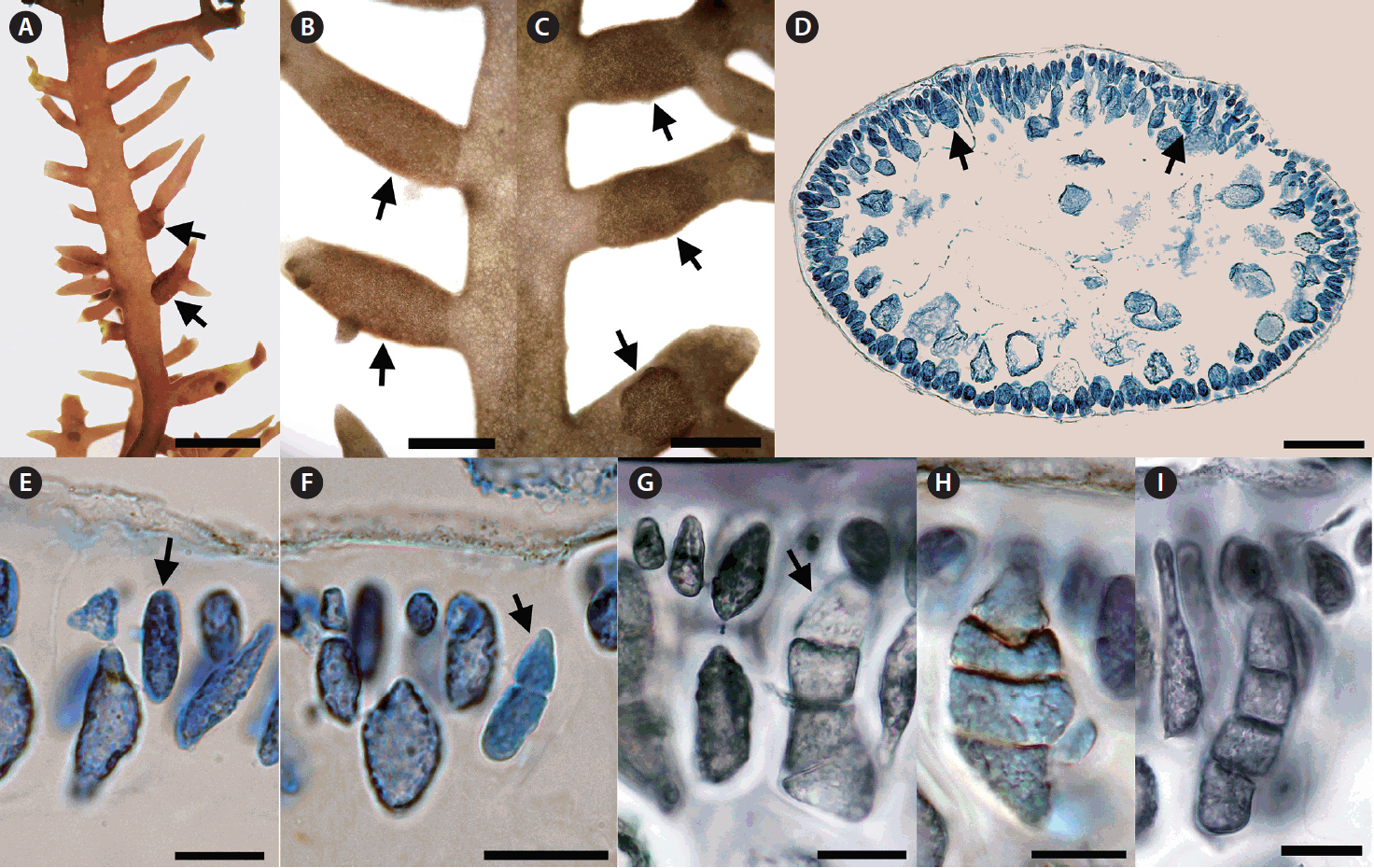
Tetrasporangia formed sori covering the basal and mid portions of short cylindrical branches (Fig. 4A–C). Tetrasporangia were initiated from modified cortical cells which bore lateral cells connected through pit connections (Fig. 4D & E). After rounds of successive zonate division, tetrasporangia with 4 compartments appeared (Fig. 4F & G). Mature tetrasporangia were 15–30 μm in diameter and 60–70 μm in length (Fig. 4H & I).
Molecular phylogeny of Callblepharis saidana
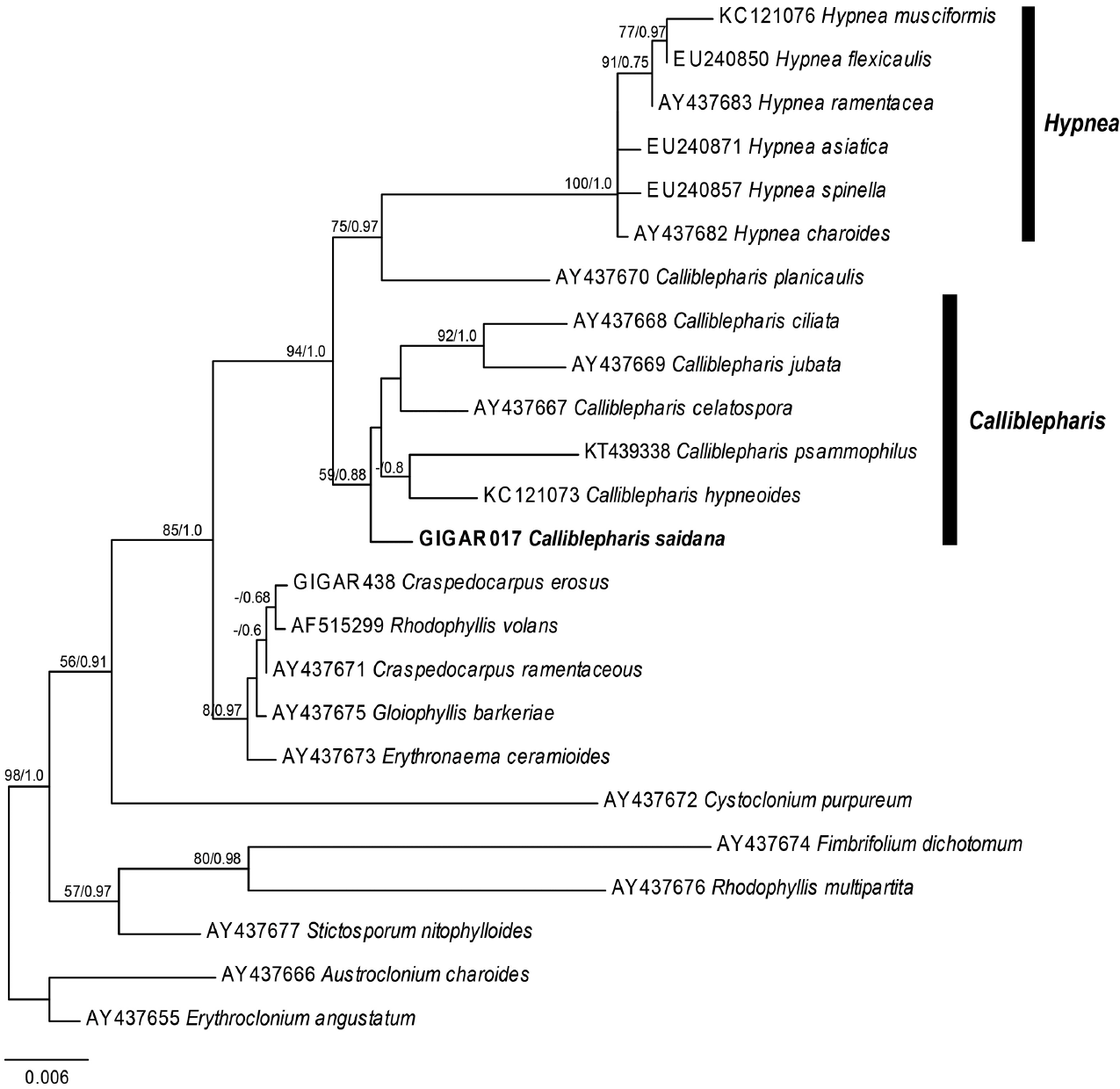
Our nuclear SSU sequence dataset included 24 sequences from 10 genera of the Cystocloniaceae and two outgroup genera of the order Gigartinales (Fig. 5). The ML and BI trees based on SSU sequences were identical and two specimens of C. saidana collected in Korea and Japan were also identical. The clade that included Hypnea was strongly supported (MLBt = 100/BPP = 1.0), but Calliblepharis was not monophyletic. To our surprise, C. saidana formed a group with five species of Calliblepharis, including the type species C. ciliata (AY437668), support values = 59/0.88. Interestingly, C. planicaulis (AY437670) was more closely related to the Hypnea clade, support values = 75/0.97 than to Callblepharis.
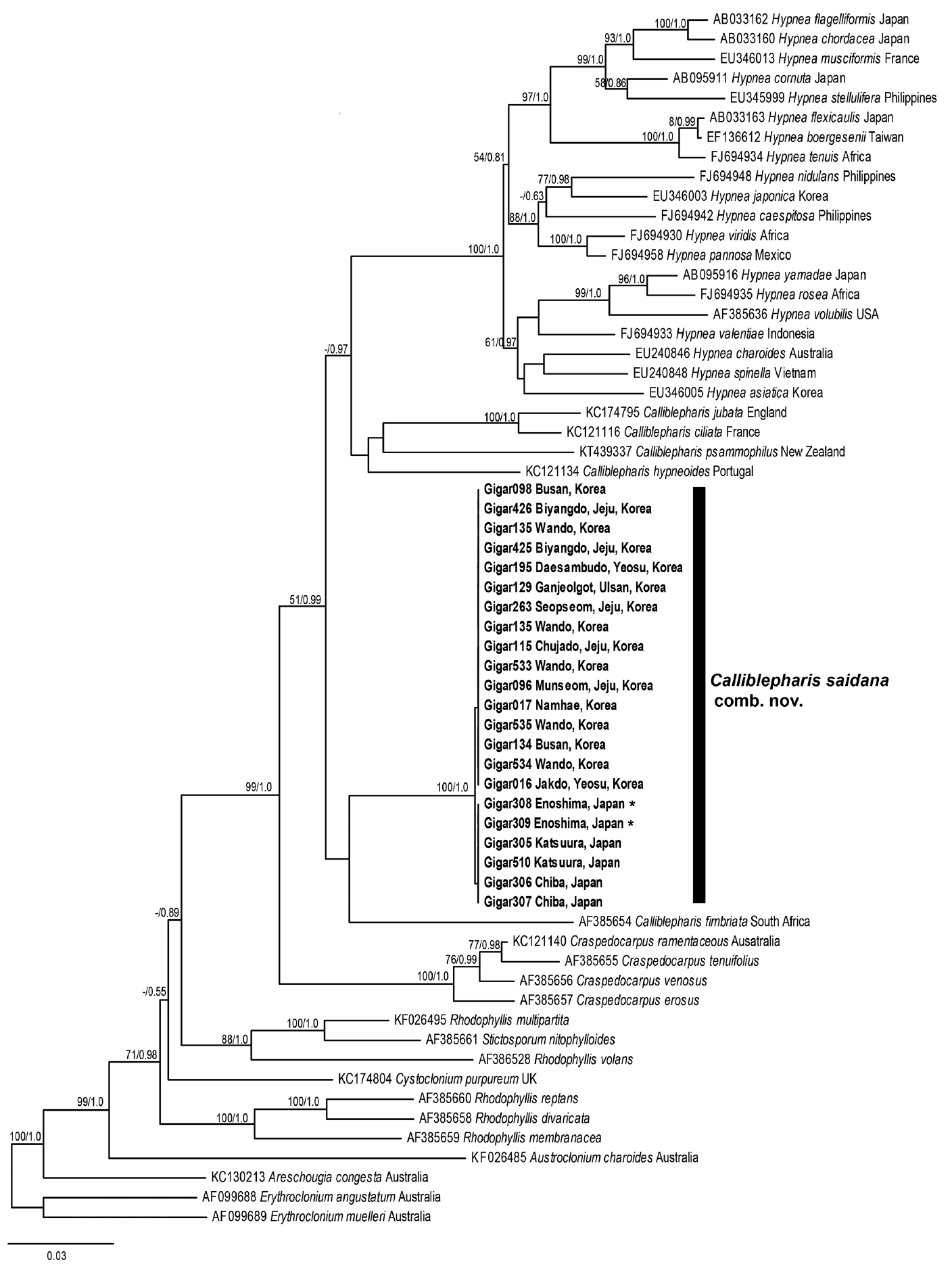
The plastid rbcL sequence dataset comprised 41 sequences representing six genera from the Cystocloniaceae and three outgroup genera from the Areschoiugiaceae of the order Gigartinales. The ML and BI trees constructed from rbcL sequences of the Cystocloniaceae were also identical (Fig. 6). All rbcL sequences from 21 specimens of C. saidana collected at 15 sites around Korea and Japan were identical as well. Twenty species of Hypnea were well clustered, but C. saidana was not included in the Hypnea clade again. Rather, C. saidana was consistently placed between C. fimbriata (AF385654) and a clade consisted four other Calliblepharis species with the type species C. cilata (KC121116).
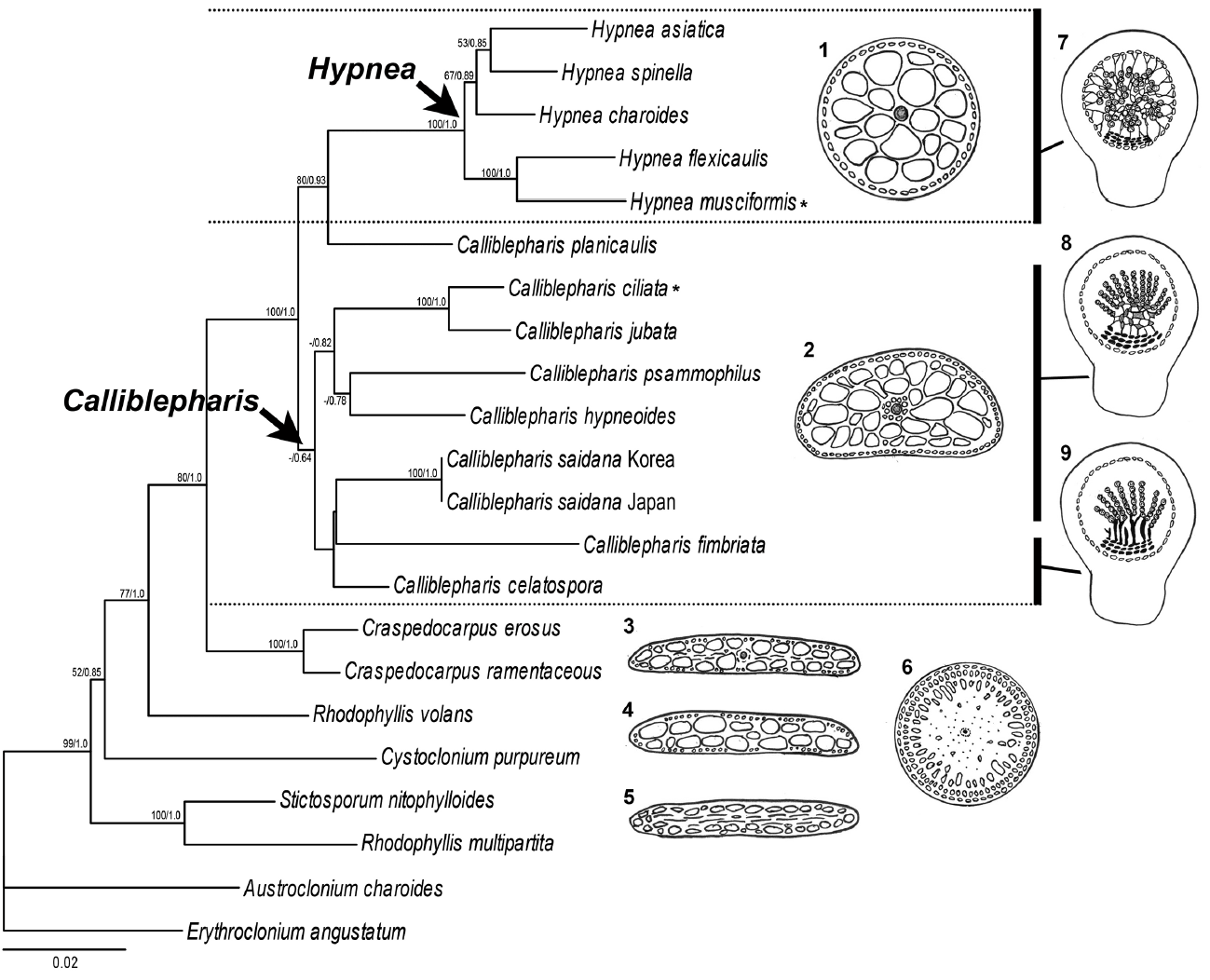
The ML and BI using the combined alignment of SSU and rbcL sequences yielded almost concordant tree, with little difference at node (Fig. 7). The combined tree resolved monophyletic node of the genus Calliblepharis including type species, C. ciliata, even though the node was supported by low bootstrap values. C. saidana formed weak sister relationships with C. fimbriata and C. celatospora.
Based on our morphological and molecular data, we conclude C. saidana is more closely related to the genus Calliblepharis than to the genus Hypnea and propose a new combination:
Calliblepharis saidana (Holmes) M. Y. Yang & M. S. Kim comb. nov
Basionym
Hypnea saidana Holmes 1895: 256 [New marine algae from Japan. J. Linn. Soc. Lond. Bot. 31:248–260, 6 plates].
DISCUSSION
Calliblepharis saidana has been assigned to the genus Hypnea since its first description because it resembles members of the genus Hypnea superficially: it has uniaxial, cartilaginous thalli, alternate branches with short branchlets, rounded medullary cells surrounding the central axial filaments, and zonately divided tetrasporangia (Holmes 1895, Okamura 1909, Tanaka 1941). However, with the continued refinement of conventional morphology-based taxonomic approach and the introduction of novel molecular analysis-based methodology, the red algal taxonomy, especially between Hypnea and Calliblepharis of the order Gigartinales, has undergone substantial restructuring recently and the need for reassessing C. saidana has been raised. For instance, the feature of a complanate thallus in vegetative morphology suggested a relationship with Calliblepharis which typically has compressed or flattened thallus (Min-Thein and Womersley 1976, Shin and Boo 1994). Furthermore, there were reports on Calliblepharis species having completely terete thallus as well: C. hypneoides and C. psammophilus collected from the Atlantic Iberian Peninsula and New Zealand, respectively (Díaz-Tapia et al. 2013, D’Archino et al. 2015).
Our molecular analyses using sequences of both SSU and rbcL demonstrated the phylogenetic position of C. saidana within the family Cystocloniaceae. The combined tree derived from SSU and rbcL alignments showed that C. saidana was grouped with the clade including the type species C. ciliata. They shared inner structure, an axial filament with surrounding elongated periaxial cells (Díaz-Tapia et al. 2013). The structure such as periaxial filaments of main axis is a synapomorphic feature of the genus Calliblepharis but it is absent in other genera of Cystocloniaceae as well as most species of Hypnea (Table 1) (Kylin 1930, Tanaka 1941, Díaz-Tapia et al. 2013). Other genera such as Craspedocarpus, Rhodophyllis, Cystoclonium, and Stictosporum have an axial cell surrounding by medullary cell and frequently have rhizoidal filaments in medulla (Fig. 7) (Min-Thein and Womersley 1976). The rhizoidal filaments are absent in both genera Calliblepharis and Hypnea. The genus Craspedocarpus is similar with Calliblepharis in having several periaxial filaments surrounding axial cell, but Craspedocarpus differs by its rhizoids in the medulla and well developed rosette in cortical cells (Womersley 1994).
Contrary to thallus morphology, female reproductive structures reliably discriminate Calliblepharis from Hypnea despite their convoluted taxonomic history. Both genera were originally placed in the family Hypneaceae because of the procarp structure (Kylin 1930). Calliblepharis was later moved to the family Cystocloniaceae (as Rhodophyllidaceae) based on the female reproductive structures (Kylin 1932). However, Hypneaceae and Cystocloniaceae were recently merged based on the phylogenetic analysis of SSU sequences (Saunders et al. 2004), putting Calliblepharis and Hypnea in the same family again. Notwithstanding, Calliblepharis is readily distinguished from Hypnea by its female structures (Fig. 7) (Kylin 1928, 1930) and by its molecular phylogeny (Díaz-Tapia et al. 2013, D’Archino et al. 2015). The central clusters of cystocarps in Calliblepharis congeners either form a reticulum or arrange themselves into parallel columns (Chiovitti et al. 1998, Díaz-Tapia et al. 2013). C. saidana and the type species C. ciliata (Díaz-Tapia et al. 2013) have reticula, but Hypnea congeners lack this structure. On the other hand, central clusters of cells without large fusion cells have been observed previously in Calliblepharis and Hypnea (Hommersand and Fredericq 1990, Chiovitti et al. 1998, Díaz-Tapia et al. 2013, Vázquez-Delfín et al. 2016). The clusters of small cells produce radial gonimoblast filaments, each of which bears only one terminal carposporangium in Hypnea (Kylin 1930, Vázquez-Delfín et al. 2016), while that in Calliblepharis bears a chain of carposporangia at the end as shown in this study for C. saidana. Finally, gonimoblasts generate elongated filaments attached to the pericarp and placenta (Kylin 1930, Vázquez-Delfín et al. 2016) in Hypnea while no such cells were observed in Calliblepharis. C. psammophilus is an interesting exception in that it possesses a central fusion cell develops chains of gonimoblast filaments (D’Archino et al. 2015). The post-fertilization events of C. psammophilus should be further analyzed in detail. In the combined tree, C. saidana was sister with C. fimbriata and C. celatospora even though the clade was weakly supported. These two sister species have gonimoblasts in a series of parallel column oriented placenta in distinct from C. saidana and C. ciliata (Chiovitti et al. 1998). This gonimoblast characteristics were considered the possibility for creating a new genus (Chiovitti et al. 1998), but it was still unresolved in this study, meaning that a wider taxon sampling is necessary to infer the phylogenetic relationships in the genus Calliblepharis. Interestingly, Calliblepharis planicaulis was positioned between two genera, Calliblepharis and Hypnea, as found previously (Saunders et al. 2004, Díaz-Tapia et al. 2013). Combined results of molecular data and female structures of C. planicaulis (chains of carposporangia in Calliblepharis-type and filaments between gonimoblast and pericarp in Hypnea-type) (Min-Thein and Womersley 1976) were raised an intriguing taxonomic questions about generic relationship which is required additional discussion in future study.
The male structure of C. saidana was quite distinct from those of its congeners (Table 2). C. saidana formed spermatangial sori on branchlets. The spermatangia cleaved off from a spermatangial mother cell that had differentiated from a cortical cell, and formed transversely divided spermatial filaments of up to seven rounded cells. This is longer than those of C. hypneoides and C. psammophilus (Díaz-Tapia et al. 2013, D’Archino et al. 2015). C. saidana exhibited distinctive antheridial walls in the mature spermatangium. This lead to the accumulation of spermatia in one place before their release in C. saidana, which is the first observation in the family Cystocloniaceae. Piled up spermatia of C. saidana were then released en masse from the antheridial wall unlike other species in Cystolonicaceae where single spermatium was released (Min-Thein and Womersley 1976, Díaz-Tapia et al. 2013, D’Archino et al. 2015).
Gametophytes of Hypnea have rarely been found in the wild. Reis and Yoneshigue-Valentin (2000) even claimed that sexual reproduction in these plants has been replaced by asexual and vegetative forms of reproduction, perhaps as a strategy, to survive under difficult environmental conditions. We were very fortunate to collect specimens having female and male reproductive structures that provided morphological evidence supporting our molecular analysis. Our combination of morphological and molecular approaches strongly suggested new taxonomic entity that can extend the understanding of this group. Apparently, further information on the reproductive structures and molecular studies of other species in Calliblepharis are required to improve our knowledge on the phylogenetic relationships within this group. Introducing C. saidana in this study, we expand the range of Calliblepharis habitat to the northwestern Pacific (Korea and Japan) from the eastern Atlantic, South Africa, the northern Indian Ocean, Australasia, and Brazil.