INTRODUCTION
Ecophysiological traits characterize individual algal species and their response patterns to abiotic and biotic factors (Lüning 1990), and are based on the concept of the fundamental niche versus the realized niche after Hutchinson (1957). Hutchinson (1957) considered the niche as an array of biotic and abiotic parameters under which a species is able to persist and maintain population sizes. This ecological concept was at the beginning restricted to terrestrial organisms, but later transferred also to marine seaweeds (Marcelino and Verbruggen 2015). The latter authors reviewed ecological niche models for invasive seaweeds and highlight this important approach to better understand and monitor whether invasive species are likely to establish and spread in a new habitat.
In the intertidal zone macroalgae have to tolerate changing and fluctuating environmental factors such as light, temperature, salinity and mechanical forces (Lüning 1990, Davison and Pearson 1996). Major problems intertidal seaweeds have to cope with are diurnal and seasonal changes of these abiotic factors (Davison and Pearson 1996, Bischof et al. 2006, Diehl et al. 2019). Salinity in intertidal zones is one of the key stressors which is influenced by tides, hydrological conditions, wind, precipitation and evaporation (Karsten 2012). During low tides intertidal macroalgae are exposed to hypo- or hypersaline conditions (Kirst 1990). Hyposaline conditions are caused by rain, snow or melt water (Karsten 2012). In contrast, hypersaline conditions emerge through evaporation during summer or freezing-out of freshwater during winter (Karsten 2012). As a result macroalgae living in the intertidal zone have developed various protective mechanisms (Kirst 1990, Karsten 2012, Scherner et al. 2013, van Ginneken 2018). Protective mechanisms at the physiological level include osmotic acclimation, which reflects the salinity tolerance of macroalgae (Kirst 1990). Photosynthesis and respiration are strongly affected by salinity changes (Sudhir and Murthy 2004, Scherner et al. 2013). High salinities typically inhibit at least three sites in the photosynthetic machinery: (1) photoactivation of electron flow on the reducing site of photosystem I, (2) electron flow on the water splitting site of photosystem II, (3) transfer of light energy between pigment complexes (Kirst 1990). Physiological parameters such as the rates of survival, growth, photosynthesis, respiration and reproduction are used to quantify the range of salinity tolerance of macroalgae (Kirst 1990, Eggert et al. 2007, Fredersdorf et al. 2009). The shape of the growth or photosynthesis rate curve (narrow versus wide) in various salinities characterizes algal species as steno- or euryhaline (Karsten 2012). Euryhaline macroalgae are typically eulittoral species, while stenohaline species are mainly sublittoral (Russell 1987). Osmolytes, low-molecular-weight organic solutes, are synthesized and accumulated or degraded under salinity stress (Ben-Amotz and Avron 1983, Angell et al 2015). Additionally, organic osmolytes exert further biochemical functions as they can act as compatible solutes (protect enzymes and structural molecules), i.e., these compounds are highly soluble in the cytoplasm and non-toxic at high concentrations (Kirst 1990, Shetty et al. 2019). In the last decades much progress has been made in identifying low-molecular-weight carbohydrates, especially in red algae (Karsten 2012), and compounds such as floridoside, mannitol, dulcitol, sorbitol and trehalose have been reported (Karsten et al. 1997, Eggert and Karsten 2010). Many species within the Florideophyceae typically contain floridoside (Karsten et al. 2007). Species within the order Ceramiales synthesize digeneaside instead of floridoside, and some genera like Bostrychia additionally synthesize polyols such as sorbitol, dulcitol, or even mannitol (e.g., Caloglossa) (Karsten et al. 2005, 2007).
New Zealand’s coastline has a rich diversity of macroalgae, especially red algae, but their ecophysiological traits are poorly studied (Adams 1994, Hurd et al. 2004, Nelson 2013). Few studies have been conducted on stress response patterns within intertidal macroalgae in New Zealand (Lamare et al. 2004, Schweikert et al. 2011, Muangmai et al. 2015, Bollen et al. 2016, Diehl et al. 2019). Diehl et al. (2019), for example, investigated seasonal changes on stress metabolites (UV-absorbing mycosporine-like amino acids and heterosides) in the intertidal red alga Pyropia plicata W. A. Nelson, and they reported consistently high amounts of all analysed compounds which mitigate against environmental changes typical of the intertidal zone. Growth rates of cryptic species of Bostrychia intricata (Bory) Montagne in respect of salinity and temperature were also studied (Muangmai et al. 2015). These authors showed that all strains grew at the full range of salinities and temperatures tested, but revealed strong difference in their specific growth rates. Bollen et al. (2016) investigated the salinity and temperature tolerance of two native New Zealand brown macroalgae Lessonia variegata J. Agardh and Ecklonia radiata (C. Agardh) J. Agardh in comparison to the introduced species Undaria pinnatifida (Harvey) Suringar, and Undaria displayed broader tolerance to the experimental stressors than native kelps.
Many macroalgae in New Zealand are indigenous (Adams 1994, Nelson 1994, Nelson et al. 2013) but non-indigenous taxa continue to arrive due to intense ship traffic, changing environmental conditions and increased monitoring (D’Archino and Zuccarello 2014, 2021). Invasive species are often more tolerant to abiotic conditions than native ones (Bollen et al. 2016). In the last few years, many introduced macroalgal species have been reported in New Zealand, such as the red alga Schizymenia apoda (J. Agardh) J. Agardh (D’Archino et al. 2007, 2015, Nelson et al. 2013, D’Archino and Zuccarello 2014, 2021, Garbary et al. 2020), but their establishment, spread and ecophysiological traits in the new habitat are mainly unknown.
The present study compared two native red algal species with two introduced red algae in respect of their salinity tolerance. Bostrychia arbuscula W. H. Harvey (Ceramiales, Rhodomelaceae) and Champia novae-zelandiae (J. D. Hooker & Harvey) Harvey (Rhodymeniales, Champiaceae) are native to New Zealand and distributed on the North Island, the South Island, the Chatham Islands, and Stewart Island (Nelson 2013). Bostrychia species as intertidal to supralittoral macroalgae are described as euryhaline, as many studies indicate their broad salinity tolerance (Karsten et al. 1995, 1996a, Muangmai et al. 2015). Champia novae-zelandiae is found from the intertidal to the upper subtidal (Nelson 2013), and based on its zonation expected to be less euryhaline compared to Bostrychia. The introduced species belongs to the genus Schizymenia (Nemastomatales, Schizymeniaceae) with currently 10 taxonomically accepted species (Guiry and Guiry 2020). Schizymenia apoda (J. Agardh) J. Agardh and S. dubyi (Chauvin ex Duby) J. Agardh are both known from different locations around the world (Gabriel et al. 2011, Ramirez et al. 2012, Saunders et al. 2015, Gunnarsson et al. 2020). In 2014, S. apoda was first noted as an introduced species to New Zealand (D’Archino and Zuccarello 2014), and more recently S. dubyi has been discovered in Wellington Harbour (D’Archino and Zuccarello 2020, and unpublished observations). Both taxa are mainly found in the low intertidal to shallow subtidal and in large tidal pools (Guiry and Guiry 2020).
The aim of this study was to compare the salinity tolerance of the two native and the introduced species to better understand the respective response patterns with an emphasis on the function of organic osmolytes. The hypothesis that B. arbuscula and C. novae-zelandiae as native species are well adapted to their intertidal habitat in New Zealand with highly fluctuating salinities was tested. Hence, it was expected that both native species show a higher salt tolerance than the introduced Schizymenia spp.
MATERIALS AND METHODS
Species collections
All three algae (Bostrychia arbuscula, Champia novae-zelandiae and Schizymenia spp.) were collected in winter from different collection spots for the salt stress experiments. On the date of collection the salinity was measured, using a Pocket Refractometer (PAL-06S; Atago, Tokyo, Japan). An overview of the location, the date of collection and the salinity at each collection spot is shown in the Supplementary Table S1. More details on the collection areas and the abiotic data are found in Gambichler et al. (2021).
For the salinity experiment, we had to deal with the issue of distinguishing the two Schizymenia species (S. apoda and S. dubyi) morphologically, which is very difficult, so a molecular survey was done to identify both taxa in the Whairepo Lagoon (Gambichler et al. 2021). Based on the similarity in the biochemical profiles and due to the taxonomic difficulty of separating both taxa (Gambichler et al. 2021), the data used in the present study is a mix of both Schizymenia spp.
Experimental set up
All specimens were brought to the laboratory, soon after collection, and acclimated for 24 h in seawater (38 SA) with aeration, light (photoperiod 12 h light-dark cycle, irradiance: 55 ± 5 μmoL photons m−2 s−1) and temperature (14 ± 1°C) conditions. The salt stress experiments were performed for 5 days. During the experiment the cleaned specimens were stored in 200 mL plastic containers filled with sterile seawater of seven different salinities (absolute salinity: 1, 5, 15, 20, 38, 45, and 60 SA) with three replicates for each salinity. The different salinities were prepared by either diluting seawater with ultrapure water (dH2O) or by adding sea salt (Aquaforest, Poland) to increase salinities. Additionally 1 mL of 1 mM NaHCO3, 5 mL of modified Provasoli’s medium (PES) (after West and McBride 1999) and 200 μL of GeO2 (1 g mL−1) were added to 1 L of media to prevent diatom growth and ensure the availability of sufficient bicarbonate for photosynthesis. The medium was changed on the second day. At the end of the experiment all samples were oven dried (60°C) and stored in the dark until further analyses. The organic osmolytes in the dried algal samples were measured with high performance liquid chromatography (HPLC 1260 Infinity System; Agilent Technologies GmbH, Waldbronn, Germany). A detailed description of the extraction method and the measurement of the organic osmolytes are found in Gambichler et al. (2021).
The in vivo chlorophyll a fluorescence of each sample and an in situ sample without treatment were measured before the start of the experiment (day 1) and every day during the salt stress experiments until day 5, with an underwater pulse-amplitude-modulated fluorometer (Diving-PAM; Walz GmbH Mess- und Regeltechnik, Effeltrich, Germany). The maximum quantum yield (Fv/Fm) was always measured after 10 min of dark acclimation and calculated with the fluorescence maximum (Fm) and F0 the minimum level of fluorescence (Fv/Fm = (Fm − F0)/Fm). All values were calculated and processed in Excel 2010. Detailed tables of all 5-day measurement series for all three algae are provided in the Supplementary Tables S2–S4. The Fv/Fm represents an indicator of the photosynthetic performance of the alga and is often affected under stress exposure (Maxwell and Johnson 2000). The PAM set up was determined for every algal species individually (see Supplementary Table S5).
Statistics
All statistical analyses were performed using SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). To test for interaction effects between the Fv/Fm and the salinity a mixed ANOVA (General linear model with repeated measures) with one between-subject (treatment = salinity as independent variable) and one within-subject (day = start Fv/Fm and end Fv/Fm as dependent variable) was performed. To analyse significant differences between the organic osmolytes concentration and the salinity, a one-way ANOVA was performed for each alga with the organic osmolyte content as the dependent variable and salinity as the independent variable (7 levels). Depending if the assumptions were met (normality with the Shapiro-Wilk Test and Q-Q-Plots, outliers with a boxplot, homogeneity of variances with Levene’s test at p > 0.05), the appropriate tests were interpreted. The violation of normality in some cases were neglected due to the small sample and replicate sizes (Underwood 1997). If the ANOVA or Welch-ANOVA (for violation of Levene’s test) showed significant differences (p < 0.05), a post-hoc Tukey’s honestly significant difference or a Games-Howell post-hoc test was analysed for more detailed interpretation and case lettering of the graphs. All statistical results are found in detail in the Supplementary Tables S6–S11.
RESULTS
Photosynthetic activity
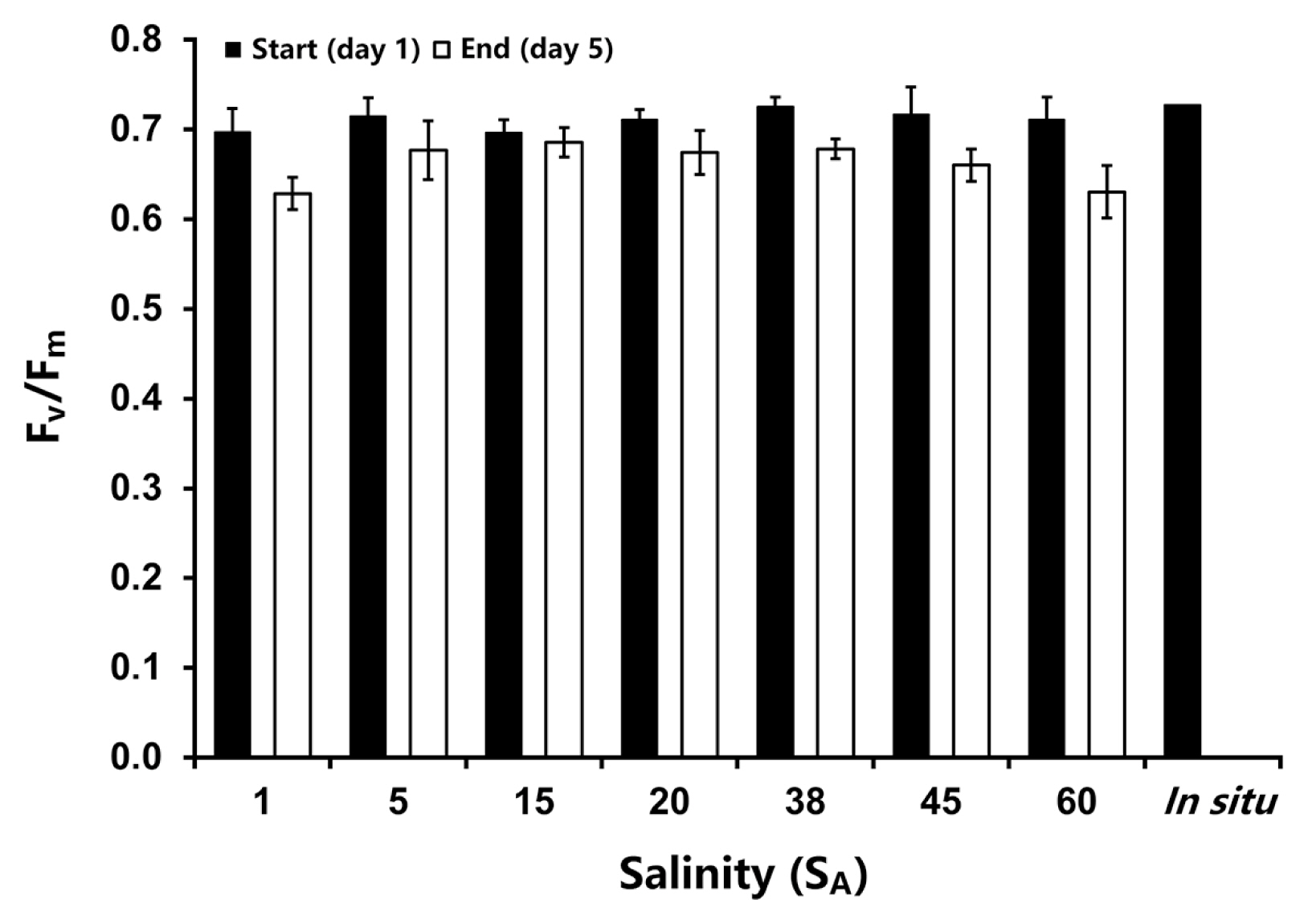
Bostrychia arbuscula exhibited high initial Fv/Fm values between 0.70 and 0.73. Exposure to different salt concentrations led to only minor decreases in the Fv/Fm, in the range of 0.63 to 0.69, whereby the strongest effect could be observed at the lowest (1 SA) and the highest (60 SA) salinity tested (mixed ANOVA: F(1,14) = 30.69, p < 0.05) (Fig. 1). There was no significantly difference found between the treatments but the Fv/Fm values between day 1 and 5 were significantly different. All statistical details can be found in the Supplementary Table S6.
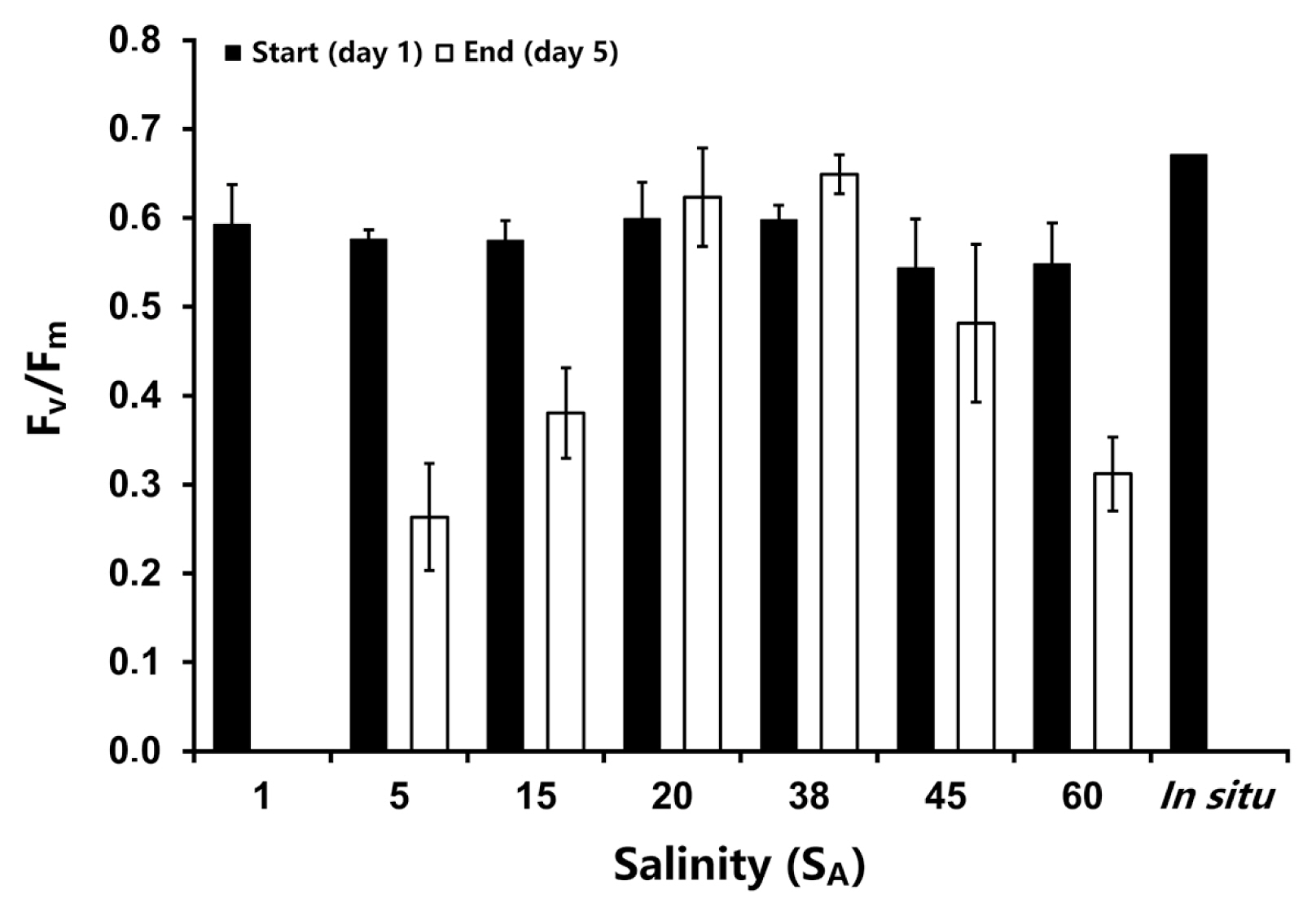
Compared to B. arbuscula, the Fv/Fm values in Champia novae-zelandiae were slightly lower in the in situ sample (0.67) and the thalli at the beginning of the salt experiment (0.54–0.60) (Fig. 2). Salinity had a very strong effect on Fv/Fm after 5 days of treatment (ANOVA: F(6,20) = 36.708, p < 0.05) (see Supplementary Table S7). At 1 SA in vivo chlorophyll a fluorescence could not be measured, and at 20 SA and 38 SA, the optimum salinities, the values of Fv/Fm at day 5 were slightly higher than those at the beginning (Fig. 2). Hyposaline conditions (5 and 15 SA) were accompanied by a strong decline in Fv/Fm and also exposure to hypersaline media of 45 and 60 SA led to decreasing Fv/Fm values.
Schizymenia spp. exhibited the narrowest salinity tolerance of the taxa tested as the Fv/Fm was completed inhibited at 1 and 5 SA (Fig. 3), as reflected in bleached and hence dead thalli. The highest Fv/Fm value was at 38 SA and hypo- and hypersaline conditions exposure led both to strongly declining photosynthetic activity (Welch-ANOVA: F(4,4.709) = 13.575, p < 0.05). The start values of Fv/Fm were similar, ranging between 0.51 ± 0.05 and 0.65 ± 0.07. After 5 days, the Fv/Fm of the treatments 15 and 60 SA decreased strongly compared to the yield values at day 1 (Fig. 3, Supplementary Tables S4 & S8 for statistical details).
Organic osmolytes
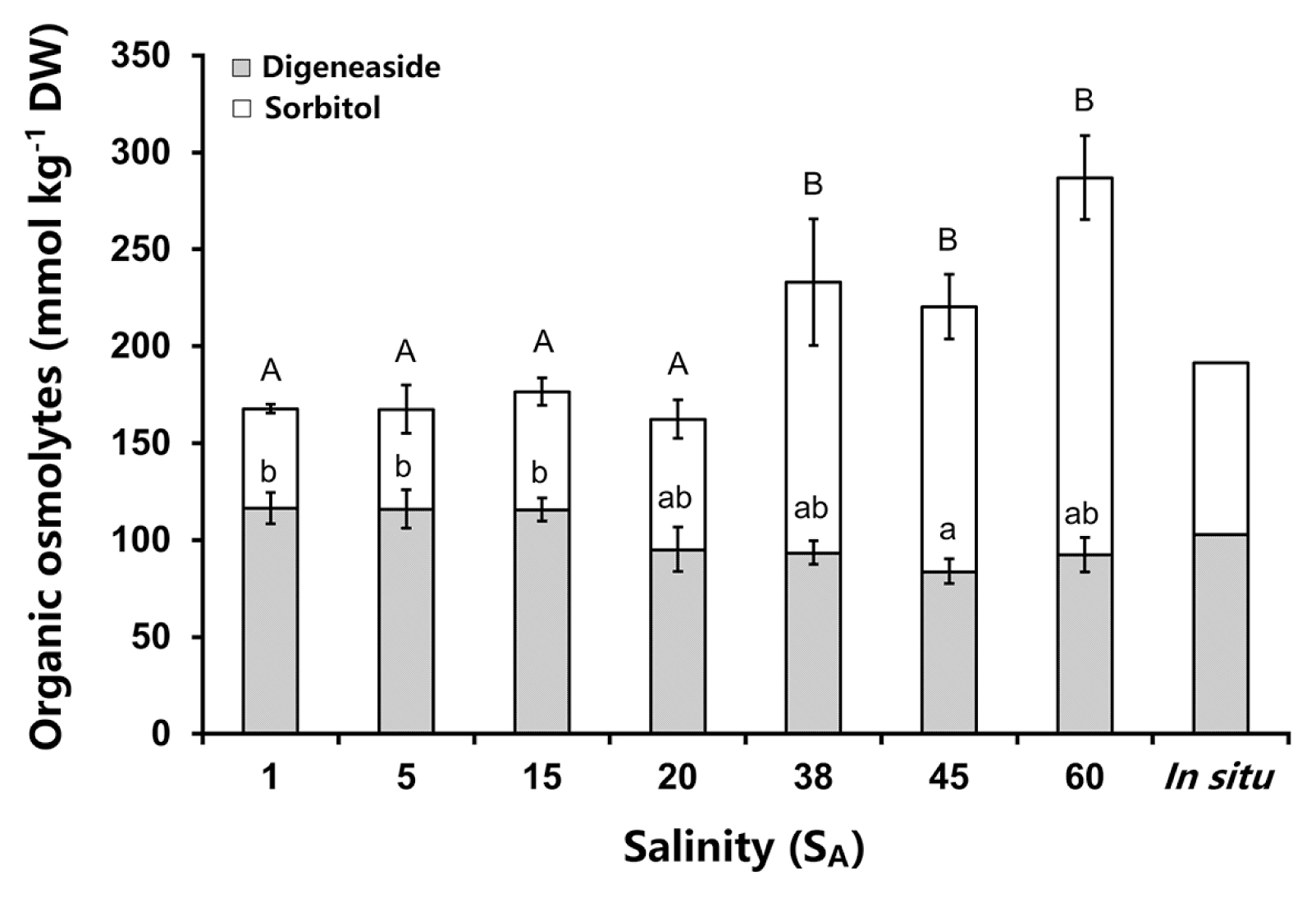
Bostrychia arbuscula contained as the main organic osmolytes the polyol sorbitol and the heteroside digenaside (Fig. 4). The total concentrations of both osmolytes changed significantly between the different salinity treatments (one-way ANOVA: F(6,14) = 8.263, p < 0.05). The total organic osmolyte contents increased with higher salinity from 166 to 287 mmol kg−1 DW (Fig. 4). However, both osmolytes showed different response patterns in B. arbuscula, as the digeneaside concentration had lower variation then that of sorbitol. The digeneaside amounts were slightly higher in the hyposaline treatments (1 to 15 SA) with about 116 mmol kg−1 DW than under hypersaline conditions (45 and 60 SA) with approximately 90 mmol kg−1 DW (Fig. 4). For the in situ sample a digeneaside content of 103 mmol kg−1 DW was measured. The sorbitol values increased with higher salinities and ranged from 50 to 195 mmol kg−1 DW (Fig. 4). The highest sorbitol amounts with 140 up to 200 mmol kg−1 DW were measured at higher salinities (38, 45, and 60 SA) and these were significantly different to the lower sorbitol concentrations between 1 and 20 SA (Fig. 4). The sorbitol level of the in situ sample was relatively low, 89 mmol kg−1 DW. Overall the proportion of sorbitol to digeneaside changed at the higher salinities. Digeneaside dominated sorbitol in the hyposaline media (approx. 56 : 44) while under hypersaline conditions sorbitol dominated digeneaside (approx. 64 : 36). All statistical details to the significant differences in Fig. 4 can be found in the Supplementary Table S9.
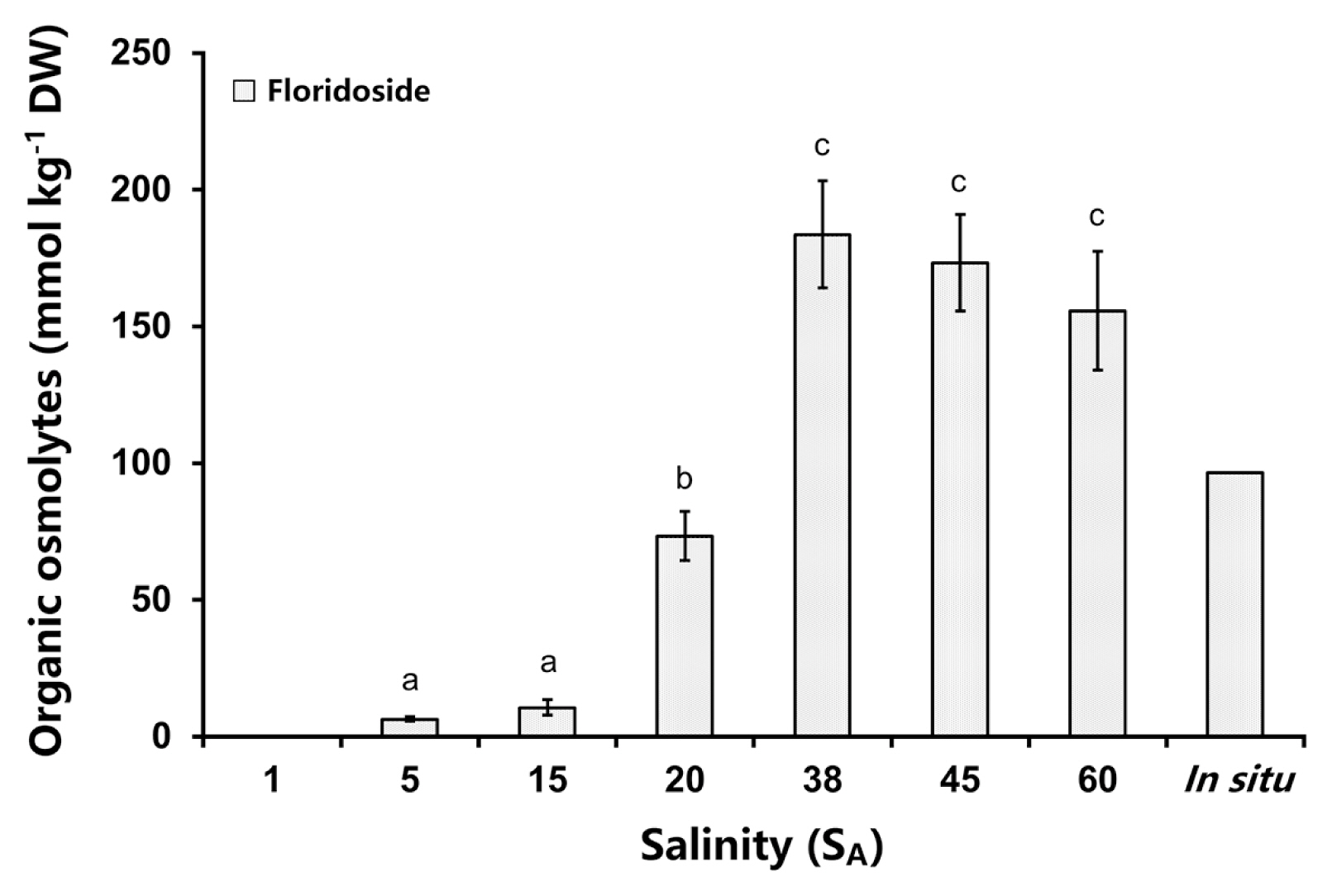
In the in situ sample and in the samples from the salt experiment of C. novae-zelandiae the heteroside floridoside was identified as the only organic osmolyte (Fig. 5). The concentrations of floridoside changed significantly between the different salinities (one-way ANOVA: F(5,12) = 62.831, p < 0.05) (Supplementary Table S10). The highest floridoside concentration was measured at 38 SA (184 mmol kg−1 DW) but this was not significantly different to amounts at 45 SA (173 mmol kg−1 DW) and 60 SA (156 mmol kg−1 DW). Reversely, the floridoside content strongly decreased from 38 to 15 SA and 5 SA hyposaline treatments (Fig. 5). The two lowest amounts of floridoside at 5 SA (6 mmol kg−1 DW) and SA 15 (11 mmol kg−1 DW) were significantly different to all other salinities (p < 0.05) (Supplementary Table S7). The in situ sample showed a similar floridoside content (97 mmol kg−1 DW) to those at 20 SA (73 mmol kg−1 DW) (Supplementary Table S10).
Floridoside was also the main organic osmolyte in Schizymenia spp. after the 5-day incubation and in the in situ sample (Fig. 6). The contents of floridoside increased continuously from hyposaline to hypersaline media (Fig. 6). The two lowest values of floridoside at 15 SA (22 mmol kg−1 DW) and 20 SA (33 mmol kg−1 DW) which were significantly different to the two highest amounts of floridoside at 45 SA (146 mmol kg−1 DW) and 60 SA (162 mmol kg−1 DW) (Welch-ANOVA: F(4,4.855) = 169.78, p < 0.05) (Supplementary Table S11). The samples of 1 SA and 5 SA were not tested for floridoside concentrations as no in vivo chlorophyll a fluorescence was measured (Figs 3 & 6).
Besides floridoside, an additional, chemically unknown compound was detected during HPLC analysis in Schizymenia spp. (Gambichler et al. 2021). This unknown compound varied between the different salinities (Supplementary Fig. S1). In hyposaline treatments (15 and 20 SA) the unknown compound dominated floridoside making up >50% of both compounds, while under normal salinity and hypersaline conditions the percentage proportion decreased (Supplementary Fig. S1).
DISCUSSION
The maximum quantum yield (Fv/Fm) was evaluated as an important parameter for characterizing the physiological status of the respective alga under salt stress (Maxwell and Johnson 2000). The in vivo chlorophyll a fluorescence measurements of Bostrychia arbuscula showed no significant decline of the Fv/Fm after a 5-day-incubation in different salinities. This indicates that B. arbuscula was neither stressed by the treatments, pointing to a broad salinity tolerance resulting in a wide fundamental niche in respect to this environmental factor. Different studies support this and describe Bostrychia species as being generally euryhaline and extremophilic (Karsten and Kirst 1989, Karsten et al. 1995, 1996a, Broderick and Dawes 1998, Muangmai et al. 2015). This broad photosynthetic tolerance was also found in other intertidal red algae, such as, for example, Bangiopsis subsimplex (Montagne) F. Schmitz from the Baltic Sea (Eggert et al. 2007). In contrast, for Pyropia plicata which is also a high intertidal species and co-occurring with B. arbuscula, a strong decrease of the Fv/Fm under extreme hypo- and hypersaline conditions was observed (Diehl et al. 2019). These euryhaline traits are supported by some earlier results on B. arbuscula, which report fast and almost complete recovery from long periods of severe desiccation (Brown 1987). Analysing the organic osmolytes in B. arbuscula after 5 days of salt stress showed a clear response, with higher concentrations under hypersaline conditions. Such findings were already reported in other studies on different intertidal algae (Eggert et al. 2007, Diehl et al. 2019). In B. arbuscula only the sorbitol concentration increased with higher salinities, while the digeneaside contents remained within a similar range between 84 and 116 mmol kg−1 DW for all salinities. A similar pattern was already reported for Bangiopsis subsimplex (Eggert et al. 2007). This supports the assumption that sorbitol is the dominant and regulated salt stress protective substance while digeneaside plays only a minor role, if at all, in osmotic adjustment (Karsten et al. 2005, 2007). The seasonal measurements of the organic osmolytes support this assumption, as the digeneaside concentrations did not show high fluctuations between the months while the sorbitol concentrations varied strongly (Gambichler et al. 2021). The rather unusual capability for some red algal genera to synthesize sorbitol has been interpreted as a biochemical key trait to cope with highly fluctuating environmental conditions such as in the high intertidal/supralittoral zone (Karsten 2012). Sorbitol and other polyols exert various physiological and biochemical functions that contribute to water-holding of cells during desiccation and to the stabilization of biomolecules under various stressors (Yancey 2005).
Champia novae-zelandiae was much less salinity tolerant compared to B. arbuscula as the in vivo chlorophyll a fluorescence data showed a strong decline under the lowest and highest salinities tested, while at 1 SA even complete inhibition was observed. Optimum quantum yield occurred at 20 and 38 SA pointing to their fundamental niche in a rather narrow range of salinities. The results of the Fv/Fm of C. novae-zelandiae are partly reflected in the results on the organic osmolyte concentrations, since at 1 SA no floridoside was detected and hence it was assumed that the specimens at the lowest salinity were dead, which was further supported by bleached thalli (unpublished observations).
Between 5 and 38 SA the floridoside amounts continuously increased with rising salinity, while under hypersaline conditions the concentrations remained unchanged, like control levels. This points to the capability of C. novae-zelandiae for osmotic acclimation only under hyposaline stress but not under hypersaline conditions. The described response pattern might be explained by the low intertidal position on the shore, which exhibits lower salinity fluctuations compared to the upper intertidal/supralittoral. The physiological role of floridoside as an organic osmolyte in red algae is well described in the literature (e.g., Kirst and Bisson 1979, Wiencke and Läuchili 1981, Kirst 1990) but also recent studies confirm that floridoside contents increase or decrease under hypersaline or hyposaline conditions, respectively (Simon-Colin et al. 2002, Lv et al. 2019). The first experimental proof of a protective function of floridoside on in vitro enzyme activity was provided by Karsten et al. (1996b). These authors showed that malate dehydrogenase and glucose-6-phosphate dehydrogenase extracted from the mangrove red alga Catenella nipae were strongly inhibited by rising NaCl concentrations up to 600 mM, while equimolar concentrations of floridoside did not inhibit enzyme activity.
Although macroalgae are capable of adjusting to new salinity conditions within a few days (Kirst 1990), it could be possible that C. novae-zelandiae relies on inorganic ions as osmolytes under hypersaline conditions. This would save energy as de novo floridoside biosynthesis is metabolically more expensive compared to ion uptake (>50 fold) (Karsten 2012). The energy requirements for ion transport across membranes in the green macroalga Ulva lactuca are equivalent to only 10–30% of the energy provided by respiration (Ritchie 1988). There exists good evidence that many inorganic ion transporters in macroalgae are generally fast and energetically cheap compared with the cost for biosynthesis of organic osmolytes (Kirst 1990).
The introduced Schizymenia spp. was even less salt tolerant then C. novae-zelandiae as reflected in complete inhibition of the Fv/Fm at both 1 and 5 SA. These data are supported by the observation that the thalli started to bleach during hyposaline treatment thereby losing pigments (data not shown). In addition, at 15 and 60 SA the Fv/Fm was also strongly inhibited. All these data clearly indicate stenohaline features of Schizymenia spp., which is in agreement with the preferential occurrence in tidal rock pools, where this taxon is mainly submerged. Like in C. novae-zelandiae, floridoside was the main organic osmolyte in Schizymenia spp., which was up-regulated between 15 and 60 SA, although there was no statistically significant difference between 45 and 60 SA. Gambichler et al. (2021) discovered in seasonally taken monthly samples of Schizymenia spp., an unknown organic compound which was also found in the present study. At low salinities (15 and 20 SA) this compound had a higher proportion than floridoside, while under hypersaline conditions its share declined. Although neither the chemical structure nor its function could be resolved yet, this unknown compound seems to play a role in osmotic acclimation of Schizymenia spp. (Gambichler et al. 2021).
This study examined the salinity tolerance of two native red algae in New Zealand and an introduced set of species, by measuring photosynthetic activity and organic osmolytes. As expected, the highest salt tolerance was found for the native high intertidal/supralittoral species B. arbuscula. In contrast, the introduced species Schizymenia spp. cannot cope with hyposaline conditions, similar to the native species C. novae-zelandiae. The different ecophysiological response patterns correlate well with the vertical distribution on the rocky shore. As the introduced Schizymenia spp. is so stenohaline, it preferentially grows in rather protected and stable microhabitats such as tide pools.
SUPPLEMENTARY MATERIALS
Supplementary Table S1. Details for all three species collected for the salt stress experiment (https://www.e-algae.org).
Supplementary Table S2. Mean (n = 3) of the in vivo chlorophyll a fluorescence of Bostrychia arbuscula during 5 days of salt treatment at 7 salinities and one in situ sample (no treatment) (https://www.e-algae.org).
Supplementary Table S3. Mean (n = 3) of the in vivo chlorophyll a fluorescence of Champia novae-zelandiae during 5 days of salt treatment at 7 salinities and one in situ sample (no treatment) (https://www.e-algae.org).
Supplementary Table S4. Mean (n = 3) of the in vivo chlorophyll a fluorescence of Schizymenia spp. during 5 days of salt treatment at 7 salinities and one in situ sample (no treatment) (https://www.e-algae.org).
Supplementary Table S5. Overview of the different pulse-amplitude-modulated fluorometer (PAM) settings measured with Diving-PAM for all three algae (https://www.e-algae.org).
Supplementary Table S6. Detailed statistical analysis of the maximum quantum yield (Fv/Fm) of the salt stress experiment of Bostrychia arbuscula (https://www.e-algae.org).
Supplementary Table S7. Detailed statistical analysis of the maximum quantum yield (Fv/Fm) of the salt stress experiment of Champia novae-zelandiae (https://www.e-algae.org).
Supplementary Table S8. Detailed statistical analysis of the maximum quantum yield (Fv/Fm) of the salt stress experiment of Schizymenia spp. (https://www.e-algae.org).
Supplementary Table S9. Detailed statistical analysis of the total organic osmolyte, digenaside, and sorbitol concentrations of Bostrychia arbuscula from the salt stress experiment (https://www.e-algae.org).
Supplementary Table S10. Detailed statistical analysis of the floridoside concentration of Champia novae-zelandiae from the salt stress experiment (https://www.e-algae.org).
Supplementary Table S11. Detailed statistical analysis of the floridoside concentration of Schizymenia spp. from the salt stress experiment (https://www.e-algae.org).
Supplementary Fig. S1. Percentage proportions of floridoside and the unknown compound of the total produced compounds of Schizymenia spp. at the end of the salt stress experiment (https://www.e-algae.org).