ABSTRACTHypoxia can indeed impact the survival of protists, which play a crucial role in marine ecosystems. To better understand the protistan community structure and species that can thrive in hypoxic waters, we collected samples from both the surface and bottom waters during the hypoxic period in Jinhae and Masan Bays and the non-hypoxic period in Jinhae Bay. Subsequently, we utilized metabarcoding techniques to identify the protistan species. During hypoxia, with dissolved oxygen concentrations of 0.8 mg L−1 in Jinhae Bay and 1.8 mg L−1 in Masan Bay within the bottom waters, the phylum Dinoflagellata exhibited the highest amplicon sequence variants richness among the identified protist phyla. Following the Dinoflagellata, Ochrophyta and Ciliophora also displayed notable presence. In hypoxic waters of Jinhae and Masan Bays, we identified a total of 36 dinoflagellate species that exhibited various trophic modes. These included one autotrophic species, 14 mixotrophic species, 9 phototrophic species with undetermined trophic modes (either autotrophic or mixotrophic), 2 kleptoplastidic species, and 10 heterotrophic species. Furthermore, the hypoxic bottom water exhibited a greater number of heterotrophic dinoflagellate species compared to the non-hypoxic surface water within the same water column or the non-hypoxic bottom water. Therefore, feeding by mixotrophic and heterotrophic dinoflagellates may be partially responsible for their dominance in terms of the number of species surviving in hypoxic waters. This study not only introduces the initial documentation of 26 dinoflagellate species surviving in hypoxic conditions but also establishes a foundation for a more comprehensive understanding of the ecophysiology of dinoflagellates in hypoxic marine environments.
INTRODUCTIONHypoxia, typically defined as oxygen levels equal to or less than 2 mg O2 L−1 or 62.5 μM, frequently results from microbial oxygen consumption during the decomposition of organic materials in marine ecosystems (Diaz 2001, Kemp et al. 2009, Sinkko et al. 2013). Over the last 50 years, the global oxygen levels in oceans have annually declined by 2%; thus, deoxygenation has become one of the critical changes in oceans (Schmidtko et al. 2017, Breitburg et al. 2018). Hypoxia can affect the ecophysiology of marine organisms, influencing factors, such as the survival, abundance, development, metabolism, growth, and reproduction (Sampaio et al. 2021). Species tolerating hypoxia may become dominant, whereas species that cannot tolerate hypoxia may disappear or decline (Nilsson and Rosenberg 1994, Riedel et al. 2012). Furthermore, hypoxia can induce alterations in the structure and function of marine ecosystems.
Marine protists encompass a diverse group of single-celled organisms, varying in cell size, and they originate from nearly all branches of the eukaryotic tree of life (Caron et al. 2012, Massana 2015). Marine protists inhabit various marine environments, from surface layers with light penetration, to deep dark zones, and sediments (Ohtsuka et al. 2015, Lim and Jeong 2021). They play diverse roles in marine ecosystems, as primary producers, prey, predators, symbiotic partners, and parasites (Azam et al. 1983, Jeong et al. 2010, Caron et al. 2012, Ok et al. 2018, 2023a). Consequently, marine protists play critical roles in marine biogeochemical cycles and food webs (Mitra et al. 2014, Edgcomb 2016). Moreover, the survival of marine protist species under hypoxic conditions may affect the structure and function of marine ecosystems. However, identifying surviving protist species using microscopy is challenging due to their relatively small size and occasional subtle morphological differences among species in the same genus.
Recently, molecular tools have been developed to efficiently identify very small protists and quantify their abundance (Gran-Stadniczeñko et al. 2019, Min and Kim 2023). Molecular sequencing is one such useful tool for characterizing protistan community structures, which identifies numerous protist taxa in target environments with high sensitivity and taxonomic resolution (Santoferrara et al. 2020, Jang et al. 2022, Kim et al. 2023). Protist community structures and some species surviving in hypoxic waters have been investigated using molecular sequencing in limited regions such as Long Island Sound, USA and Tolo Harbor, Hong Kong (Rocke et al. 2016, Santoferrara et al. 2022). To better understand protist species that can thrive in hypoxic waters and to comprehensively characterize protist community structures in various marine environments, it is essential to conduct DNA sequencing analyses across a broader range of countries and regions.
Dinoflagellates, a major group of protists in marine ecosystems, are ubiquitously present in marine environments (Gran-Stadniczeñko et al. 2019, Jeong et al. 2021, Lee et al. 2021). They play diverse ecological roles, including primary producers, prey, predators, symbionts, and parasites (Hansen 1991, Coats 1999, Stat et al. 2008, Jeong et al. 2010, Ok et al. 2022). Dinoflagellates have three major trophic modes (autotrophy, heterotrophy, and mixotrophy) and serve as various types of prey and predators (Schnepf and Elbrächter 1992, Jeong et al. 2010, Stoecker et al. 2017, Kang et al. 2023, You et al. 2023). They often form red tides or harmful algal blooms (HABs), accounting for 75% of all HAB species, resulting in extensive global damage in marine ecosystems (Smayda 1997, Hallegraeff 2004, Eom et al. 2021, Sakamoto et al. 2021, Ok et al. 2023b). Thus, understanding the ecophysiological characteristics of dinoflagellates is crucial for comprehending the structure and function of marine ecosystems. However, there is limited data available on dinoflagellate species that can thrive in hypoxic conditions. To better understand the structure and function of marine ecosystems in expanding hypoxic conditions, it is crucial to identify dinoflagellate species surviving under hypoxia and elucidate their trophic modes.
Jinhae and Masan Bays, semi-enclosed bays located in the southern part of the Korean Peninsula, are notorious for frequent annual hypoxia from May to September (Lim et al. 2006). Hypoxia in these regions is primarily caused by anthropogenic eutrophication and thermal stratification resulting from the natural sluggish water circulation (Lee et al. 2018). Although the impact of hypoxia on the macrobenthic community in these areas has been elucidated, the effects of hypoxia on the protistan community remain relatively unexplored (Lim et al. 2006); however, diverse protist species have been found in these bays (Jeong et al. 2013). Thus, these bays are ideal regions for exploring the effects of hypoxia on protistan communities.
In this study, surface and bottom seawater samples were collected from Jinhae and Masan Bays during two scenarios: one during observed hypoxia in the bottom water and non-hypoxic conditions in the surface water in August 2023, and the other from Jinhae Bay in August 2022 when hypoxia was not observed in both the surface and bottom waters. Protistan community structures and species surviving under hypoxic conditions, especially dinoflagellate species, were explored using metabarcoding analyses. The present study provides a foundational understanding of protistan community structures in hypoxic environments.
MATERIALS AND METHODSSampling and analyses of hydrological propertiesOne fixed station, SNUJH, was located in Jinhae Bay near Sokcheon Harbor at a water depth of 3 m. Another fixed station, SNUMS, was located in Masan Bay near Masan Harbor at a water depth of 3 m (Fig. 1A)
The dissolved oxygen (DO), temperature, salinity, and chlorophyll-a (Chl-a) at depths of 0, 1, 2, and 3 m at each station were measured by deploying a YSI EXO 1 instrument (YSI, Yellow Springs, OH, USA) in Jinhae and Masan Bays on Aug 18, 2023 and additionally in the Jinhae Bay station on Aug 11, 2022. Irradiance in the surface water were measured using a portable illuminance meter (IM-600; Topcon, Tokyo, Japan). The unit of irradiance in the illuminance meter, Lux, was converted to μmol photon m−2 s−1 using the conversion factor provided by Thimijan and Heins (1983). The Secchi depth was measured using a Secchi disk to calculate the extinction coefficient and light intensity at each depth.
Surface waters at each station were collected using a clean bucket, whereas the bottom waters were almost simultaneously collected using a 2.5-L Niskin sampler. For environmental DNA (eDNA), samples of up to 500 mL were filtered using 25-mm glass-fiber (GF)/C membrane filters (Whatman Inc., Clifton, NJ, USA). The filtered membranes were stored in a 2.0 mL tube at −20°C and subsequently transferred to the laboratory.
For the nutrient analysis, 20 mL of seawater from each sample was filtered through a GF/F membrane filter (Whatman Inc.). In the laboratory, concentrations of nitrate plus nitrite (NO3 + NO2), ammonium (NH4), phosphate (PO4), and silicate (SiO2) were measured using a nutrient auto-analyzer system (QuAAtro; Seal Analytical GmbH, Norderstedt, Germany).
DNA extraction, sequencing, and sequence analysisThe eDNA collected on the membrane filters were extracted using the AccuPrep genomic DNA extraction kit (Bioneer, Daejeon, Korea). The extracted eDNA was quantified using a Qubit fluorometer using Quant-IT PicoGreen (Invitrogen, Waltham, MA, USA). Subsequently, the extracted eDNA was then stored at −20°C before conducting polymerase chain reaction (PCR).
The sequencing libraries were prepared according to the Illumina metagenomic sequencing library protocols (San Diego, CA, USA) to amplify target genes. The input genomic DNA (5 ng) was amplified using PCR with 5× reaction buffer, 1 mM of deoxyribonucleotide triphosphate (dNTP) mix, 500 nM of universal forward (TAReuk454FWD1; 5′-CCAGCASCYGC GGTAATTCC-3′) and reverse primers (V4 18S Next.Rev; 5′-ACTTTC GTTCTTGATYRATGA-3′) targeting the V4 region of the 18S rRNA gene for protists (Stoeck et al. 2010, Piredda et al. 2017), and Herculase II fusion DNA polymerase (Agilent Technologies, Santa Clara, CA, USA). The first run of PCR included 3 min of incubation at 95°C for heat activation, followed by 25 cycles at 95°C for 30 s, 43°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min. The resulting products were purified using AMPure beads (Agencourt Bioscience, Beverly, MA, USA). Subsequently, 10 μL each of the first PCR products was amplified again with NexteraXT Indexed Primer for the final library construction. The thermocycler settings for the second run of PCR were the same as those for the first run of PCR condition, except for 10 cycles instead of 25 cycles. AMPure beads were used to purify the products. The final purified products were quantified by quantitative PCR (qPCR) according to the qPCR Quantification Protocol Guide (KAPA Library Quantification kits for Illumina Sequencing platforms) and qualified using the TapeStation D1000 ScreenTape (Agilent Technologies, Waldbronn, Germany). The paired-end sequencing was conducted on an Illumina MiSeq platform (Illumina) at Macrogen (Seoul, Korea).
Illumina MiSeq raw data were sorted by sample using index sequences, and paired-end FASTQ files for each sample were generated. The Cutadapt 3.2 tool was used to remove the sequencing adapter sequence and F/R primer sequences of the target gene region (Martin 2011), and then forward (Read 1) and reverse sequences (Read 2) were cut to 230 and 180 bp, respectively. The DADA2 package (version 1.18.0) in R (version 4.0.3) was used to correct errors in amplicon sequencing (Callahan et al. 2016). For paired-end reads, sequences with expected errors of ≥2 were excluded. Then, each sample was denoised after establishing an error model for each batch. After assembling the paired-end sequences into a single sequence, the chimera sequence was removed using DADA2 Consensus method, and then amplicon sequence variants (ASVs) were clustered. Normalization was conducted by subsampling based on the number of sample reads with the minimum number of reads among all samples using the QIIME v. 1.9 program (Caporaso et al. 2010). BLAST+ (version 2.9.0) was performed for taxonomic assignment of each ASV sequence on the reference database (Camacho et al. 2009). If the query coverage of the best hit was less than 85% or the identity was less than 98%, the ASV was not assigned. Moreover, ASVs not assigned to specific species in the PR2 database were further annotated using the National Center for Biotechnology Information (NCBI) database. The ASVs assigned to metazoans were eliminated from the analyses. Raw read sequences were submitted to the NCBI for Biotechnology Information Short Read Archive (accession number: PRJNA1037704).
RESULTSHydrographic properties of the study areasIn Jinhae Bay on Aug 18, 2023, with increasing water depth, the DO levels decreased from 4.9 to 0.8 mg L−1 (Fig. 1B). Water temperature also decreased from 28.7 to 26.2°C with increasing water depth; however, salinity increased from 20.8 to 25.9. With increasing water depth, the irradiance decreased from 1,870 to 27 μmol photons m−2 s−1, calculated using an extinction coefficient of 1.42 m−1. Chl-a concentrations also decreased from 58.7 to 3.5 μg L−1. Moreover, the concentrations of NO3 + NO2, PO4, and SiO2 in the surface water were higher than those in the bottom water (Table 1). However, the concentration of NH4 in the surface water was lower than that in the bottom water.
In Masan Bay on Aug 18, 2023, with increasing water depth, the DO levels decreased from 6.1 to 1.8 mg L−1 (Fig. 1C). Water temperature also decreased from 28.7 to 25.0°C with increasing water depth; however, salinity increased from 17.7 to 25.8. With increasing water depth, the irradiance decreased from 896 to 30 μmol photons m−2 s−1, calculated using an extinction coefficient of 1.13 m−1. Chl-a concentrations also decreased from 34.3 to 2.8 μg L−1. The concentrations of NO3 + NO2, PO4, and SiO2 in the surface water were higher than those in the bottom water (Table 1). However, the concentration of NH4 in the surface water was lower than that in the bottom water.
In Jinhae Bay on Aug 11, 2022, with increasing water depth, the DO levels decreased from 6.5 to 3.8 mg L−1 (Fig. 1D). With increasing water depth, water temperature slightly decreased from 27.9 to 26.8°C. The salinity of the surface water, 29.5, did not largely differ from that of the bottom water, 30.9. The irradiance at the surface was 476 to 28 μmol photons m−2 s−1, calculated using an extinction coefficient of 1.8 m−1. Chl-a concentrations decreased from 18.3 to 10.6 μg L−1 with increasing water depth. The concentrations of NH4 and PO4 and in the surface water were higher than those in the bottom water (Table 1). However, the concentrations of NO3 + NO2 and SiO2 in the surface water were lower than those in the bottom water (Table 1).
Comparison of protistan community structures in the surface and bottom waters during hypoxic and non-hypoxic periodsIn all samples, an average of 55,467 reads (standard error = 4,255) per sample was obtained from the 18S rRNA gene sequencing. In a total of 1,496 ASVs, 27 phyla were annotated (Supplementary Table S1). During the hypoxic period in Jinhae Bay on Aug 18, 2023, the number of ASVs in the hypoxic bottom water, 380, was greater than that in the non-hypoxic surface water, 230 (Fig. 2). Similarly, during the hypoxic period in Masan Bay on Aug 18, 2023, the number of ASVs in the hypoxic bottom water, 412, was greater than that in the non-hypoxic surface water, 354. However, during the non-hypoxic period in Jinhae Bay on Aug 11, 2022, the number of ASVs in the non-hypoxic bottom water, 326, was lower than that in the non-hypoxic surface water, 533. Among the assigned protist phyla in both the surface and bottom waters during both the hypoxic and non-hypoxic periods, the number of ASVs of the phylum Dinoflagellata was the highest, followed by those of the phylum Ochrophyta or Ciliophora (Fig. 2).
During the hypoxic period in Jinhae Bay on Aug 18, 2023, the ASVs of all protists detected only in the hypoxic bottom water, 286 (55.4%), were greater than those detected only in non-hypoxic surface water, 136 (26.4%), or those detected in both the surface and bottom waters, 94 (18.2%) (Fig. 3). Similarly, during the hypoxic period in Masan Bay on Aug 18, 2023, the ASVs of all protists detected only in the hypoxic bottom water, 283 (44.4%), were greater than those detected only in the non-hypoxic surface water, 225 (35.3%), or in both the surface and bottom waters, 129 (20.3%). However, during the non-hypoxic period in Jinhae Bay on Aug 11, 2022, the ASVs of all protists detected only in the non-hypoxic surface water, 354 (52.1%), were greater than those detected only in the non-hypoxic bottom water, 147 (21.6%), or in both the surface and bottom waters, 179 (26.3%). The pattern of ASVs of the protists belonging to the phylum Dinoflagellata or phylum Ciliophora was similar to that of all other protists. In contrast, the pattern of ASVs of protists belonging to the phylum Ochrophyta was slightly different from that of all the protists. The ASVs of the phylum Ochrophyta during the hypoxic period in Jinhae Bay on Aug 18, 2023, were highest in both the surface and bottom waters.
During the hypoxic period in Jinhae Bay on Aug 18, 2023, in terms of the relative read abundance, both the non-hypoxic surface and hypoxic bottom waters were dominated by the phylum Dinoflagellata (77 and 36%, respectively) (Fig. 4). During the hypoxic period in Masan Bay on Aug 18, 2023, the non-hypoxic surface water was dominated by the phylum Dinoflagellata (49%), while the hypoxic bottom water was dominated by unassigned taxa (59%), followed by Dinoflagellata (15%). During the non-hypoxic period in Jinhae Bay on Aug 11, 2022, both the non-hypoxic surface and bottom waters were dominated by the phylum Ochrophyta (45 and 62%, respectively).
Dinoflagellates surviving under hypoxic conditionsThe total number of ASVs annotated as the phylum Dinoflagellata in the present study was 301. During the hypoxic period on Aug 18, 2023, the number of ASVs from the phylum Dinoflagellata in the hypoxic bottom waters of both Jinhae and Masan Bays (91 and 86, respectively) was greater than that in the non-hypoxic surface waters (67 and 66, respectively) (Fig. 2). Conversely, in Jinhae Bay, during the non-hypoxic period on Aug 11, 2022, the number of ASVs of Dinoflagellata in the non-hypoxic bottom water (84) was lower than that in the non-hypoxic surface water (119). A total of 73 dinoflagellate species were assigned from the ASV data detected in each sample (Tables 2 & 3). Among these dinoflagellate species, 36 assigned species were detected in the hypoxic waters of Jinhae and Masan Bays, including one autotrophic species, 14 mixotrophic species, nine phototrophic species with undetermined trophic modes (either autotrophic or mixotrophic), two kleptoplastidic species, and 10 heterotrophic species (Table 4).
In Jinhae Bay on Aug 18, 2023, 54 assigned dinoflagellate ASVs (29 assigned dinoflagellate species) that survived in the hypoxic bottom water consisted of one ASV assigned as the autotroph (one autotrophic species), 16 ASVs assigned as mixotrophs (11 mixotrophic species), 20 ASVs assigned as phototrophs (six phototrophic species), one ASV assigned as the kleptoplastidic taxon (one kleptoplastidic species), and 16 ASVs assigned as heterotrophs (10 heterotrophic species) (Fig. 5A & B). Whereas, 51 assigned dinoflagellate ASVs (35 assigned dinoflagellate species) that survived in the non-hypoxic surface water consisted of two ASVs assigned as autotrophs (two autotrophic species), 21 ASVs assigned as mixotrophs (14 mixotrophic species), 19 ASVs assigned as phototrophs (11 phototrophic species), one ASV assigned as the kleptoplastidic taxon (one kleptoplastidic species), and eight ASVs assigned as heterotrophs (seven heterotrophic species).
In Masan Bay, on Aug 18, 2023, 29 assigned dinoflagellate ASVs (23 assigned dinoflagellate species) that survived in the hypoxic bottom water consisted of eight ASVs assigned as mixotrophs (six mixotrophic species), 10 ASVs assigned as phototrophs (seven phototrophic species), one ASV assigned as the kleptoplastidic taxon (one kleptoplastidic species), and 10 ASVs assigned as heterotrophs (nine heterotrophic species) (Fig. 5A & B). Whereas, 46 assigned dinoflagellate ASVs (31 assigned dinoflagellate species) that survived in the non-hypoxic surface water consisted of two ASVs assigned as autotrophs (two autotrophic species), 18 ASVs assigned as mixotrophs (13 mixotrophic species), 22 ASVs assigned as phototrophs (13 phototrophic species), and four ASVs assigned as heterotrophs (three heterotrophic species).
In Jinhae Bay, on Aug 11, 2022, 34 assigned dinoflagellate ASVs (27 assigned dinoflagellate species) that survived in the non-hypoxic bottom water consisted of one ASV assigned as the autotroph (one autotrophic species), 15 ASVs assigned as mixotrophs (nine mixotrophic species), nine ASVs assigned as phototrophs (nine phototrophic species), one ASV assigned as the kleptoplastidic taxon (one kleptoplastidic species), and eight ASVs assigned as heterotrophs (seven heterotrophic species) (Fig. 5A & B). Whereas, 36 assigned dinoflagellate ASVs (35 assigned dinoflagellate species) that survived in the non-hypoxic surface water consisted of eight ASVs assigned as mixotrophs (seven mixotrophic species), 12 ASVs assigned as phototrophs (12 phototrophic species), two ASVs assigned as kleptoplastidic taxon (two kleptoplastidic species), and 14 ASVs assigned as heterotrophs (14 heterotrophic species).
The proportion of heterotrophic dinoflagellate ASVs to total dinoflagellate ASVs in hypoxic bottom waters (30% in Jinhae Bay and 34% in Masan Bay) was higher than that in non-hypoxic surface waters (16% in Jinhae Bay and 9% in Masan Bay) during the hypoxic period on Aug 18, 2023 (Fig. 5C). Conversely, the proportion in the non-hypoxic bottom water (24% in Jinhae Bay) was lower than that in the non-hypoxic surface water (39% in Jinhae Bay) during the non-hypoxic period on Aug 11, 2022 (Fig. 5C). The proportion of heterotrophic dinoflagellate species to total dinoflagellate species in hypoxic bottom waters (34% in Jinhae Bay and 39% in Masan Bay) was higher than that in non-hypoxic surface waters (20% in Jinhae Bay and 10% in Masan Bay) during the hypoxic period on Aug 18, 2023 (Fig. 5D). Conversely, the proportion in the non-hypoxic bottom water (26% in Jinhae Bay) was lower than that in the non-hypoxic surface water (40% in Jinhae Bay) during the non-hypoxic period on Aug 11, 2022 (Fig. 5D).
In the hypoxic bottom waters of Jinhae Bay and Masan Bay, a total of 67 ASVs (36 species) assigned as Dinoflagellata were detected. Among the ASVs (species), 38 ASVs (13 species) were detected in the hypoxic bottom water of Jinhae Bay, whereas 15 ASVs (7 species) were detected in the hypoxic bottom water of Masan Bay. Moreover, 14 ASVs (16 species) were detected in the hypoxic bottom waters of both Jinhae and Masan Bays (Table 5, Fig. 5E).
DISCUSSIONHydrographic properties of the study areasJinhae and Masan Bays are notorious for severe hypoxia from May to October (Lim et al. 2006). These bays face frequent red tides and HABs (Jeong et al. 2013, Ok et al. 2021b, Sakamoto et al. 2021). Red tides often generate substantial amounts of organic material, which can lead to hypoxia (Chen et al. 2021, Dias and Kurian 2022, Ratmaya et al. 2022). When the data obtained in this study were pooled, the DO concentration in the bottom waters was revealed to be inversely related to the Chl-a concentration in the surface waters (Fig. 6). Thus, the high levels of Chl-a in non-hypoxic surface waters may be partially responsible for the formation of hypoxic bottom waters in these study areas.
In these bays, hypoxia was first reported in 2023 on May 23–24 (National Institute of Fisheries Science, Korea; https://www.nifs.go.kr/board/actionBoard0023List.do). In Jinhae Bay, on Aug 16–17, 2023, two days before this study, a hypoxia event with the lowest DO concentration recorded at 0.5 mg L−1 in the bottom water had been reported (National Institute of Fisheries Science, Korea). Therefore, the protists that survived in the hypoxic bottom waters of Jinhae and Masan Bays on Aug 18, 2023, were likely to tolerate hypoxia for at least several days.
Protistan community structures in the hypoxic and non-hypoxic watersIn both Jinhae and Masan Bays, during the hypoxic period, the number of ASVs, (reflective of the species richness) in hypoxic bottom waters was greater than that in the non-hypoxic surface waters. Rocke et al. (2013) reported higher protist species richness in the hypoxic bottom waters than in the non-hypoxic surface waters in the Gulf of Mexico. Conversely, Stauffer et al. (2013) reported lower protist species richness in hypoxic surface water than in non-hypoxic surface water in the northern basin of King Harbor, Southern California Bight. Thus, hypoxia in these regions may not be the only factor affecting ASV or species richness in marine environments. In the present study, during the hypoxic period, the salinities in non-hypoxic surface waters of both Jinhae and Masan Bays were considerably lower than those in the hypoxic bottom waters, indicating an influx of freshwater. Rocke et al. (2013) also reported lower salinities in the non-hypoxic surface water than in the hypoxic bottom water in the Gulf of Mexico, consistent with the results of our study. Conversely, Stauffer et al. (2013) reported little difference in salinities between non-hypoxic and hypoxic surface waters in the northern basin of King Harbor. In general, the number of protist species tends to increase with increasing salinity within the range of 0–35 (Telesh et al. 2013). Thus, during the hypoxic period, the lower salinity of non-hypoxic surface water may partially be responsible for the lower number of ASVs in the non-hypoxic surface water than in the hypoxic bottom water. Moreover, there is a possibility that the ASVs detected in the hypoxic bottom waters in Jinhae and Masan Bays include DNA-containing detritus originating from surface waters, leading to higher numbers of ASVs in the hypoxic bottom waters than in the non-hypoxic surface waters.
Among the phyla that are ASV-assigned in the present study, the phylum Dinoflagellata exhibited the highest relative read abundance in both non-hypoxic surface and hypoxic bottom waters during the hypoxic period. Similarly, Santoferrara et al. (2022) reported that the relative read abundance of the phylum Dinoflagellata in the hypoxic water (<2 mg L−1) of Long Island Sound, USA, was the highest among the protistan phyla. Additionally, Rocke et al. (2016) reported that the relative read abundances of the phylum Dinoflagellata in the hypoxic water of Tolo Harbor, Hong Kong, were the highest and second highest. Therefore, the consistent findings of these three studies indicate that the relative read abundance of the phylum Dinoflagellata in the protistan communities in hypoxic waters was the highest. However, to confirm this hypothesis, the highest relative read abundances of protistan phyla in non-hypoxic and hypoxic waters in more regions should be investigated.
Dinoflagellates surviving in hypoxic watersThe present study found that 36 assigned dinoflagellate species survived in the hypoxic waters using metabarcoding analysis. Among these, 10 dinoflagellate species had been previously reported to be present in hypoxic waters in literature, whereas 26 dinoflagellate species were newly discovered to be present in hypoxic waters in the present study. Thus, the results of the present study largely expanded the roster of dinoflagellate species that can survive in hypoxic waters.
Among the 36 assigned dinoflagellate species that survived in hypoxic waters, 26 were mixotrophic, heterotrophic, and kleptoplastidic, capable of feeding on prey. Therefore, feeding by these dinoflagellates may partially be responsible for their dominance among all the dinoflagellate species surviving in the hypoxia waters of Jinhae and Masan Bays. In the present study, the heterotrophic dinoflagellate Gyrodinium dominans was consistently present in all samples from both surface and bottom waters of Jinhae and Masan Bays in both hypoxic and non-hypoxic periods. Similarly, Santoferrara et al. (2022) also reported a wide distribution of G. dominans in both the surface and bottom waters during hypoxic and non-hypoxic periods in Long Island Sound, USA. In addition, Eom et al. (2024) reported that G. dominans grew in hypoxic waters by feeding on algal prey in laboratory experiments, which supports our field observations. G. dominans is known to feed on the mixotrophic dinoflagellates Akashiwo sanguinea, Heterocapsa rotundata, Karenia mikimotoi, and Margalefidinium polykrikoides (Kang et al. 2020). During the study period, G. dominans co-occurred with these mixotrophic dinoflagellates in hypoxic water. Therefore, G. dominans may survive in hypoxic environments by feeding on prey that survive in these environments.
When the amounts of organic materials increase or remain constant in bottom waters, global warming can exacerbate hypoxia by intensifying the water column stratification, which prevents mixing between the oxygenated surface and hypoxic bottom waters (Rabalais et al. 2009, Bendtsen and Hansen 2013). The results of the present study clearly show that the proportion of the number of heterotrophic dinoflagellate species to the total dinoflagellate species in hypoxic bottom waters increased compared with that in non-hypoxic surface waters during the hypoxic period. Conversely, the proportion in non-hypoxic bottom water decreased compared with that in non-hypoxic surface water during the non-hypoxic period. Thus, more frequent or intensified hypoxia may alter the structure of dinoflagellate communities. Heterotrophic dinoflagellates play diverse roles, such as predators of bacteria, nanoflagellates, diatoms, other dinoflagellates, and eggs and early naupliar stages of copepods, and as prey for diverse metazoans (Strom and Buskey 1993, Jeong 1994, 1999, Sherr and Sherr 2007, Jeong et al. 2008, 2010, 2015). Therefore, hypoxia may eventually affect the structure and function of marine ecosystems.
ACKNOWLEDGEMENTSWe thank Prof. Chung Yeon Hwang at Seoul National University for valuable comments. This research was supported by the National Research Foundation funded by the Ministry of Education (NRF-2022R1A6A3A01086348) award to JHO and the National Research Foundation by the Ministry of Science and ICT (NRF-2021R1A2C1093379; NRF-RS-2023-00291696) and Korea Institute of Marine Science & Technology Promotion (KIMST) by the Ministry of Oceans and Fisheries (20230018) award to HJJ.
SUPPLEMENTARY MATERIALSSupplementary Table S1Detailed taxonomic information of the protistan communities analyzed by 18S rRNA gene amplicon sequencing during the study period (https://www.e-algae.org). Fig. 1Map of the study area (A) and vertical profiles of dissolved oxygen (DO, mg L−1), water temperature (°C), salinity, irradiance (μmol photons m−2 s−1), and chlorophyll-a (μg L−1) concentration in Jinhae Bay on Aug 18, 2023 (B), in Masan Bay on Aug 18, 2023 (C), and in Jinhae Bay on Aug 11, 2022 (D). The red-dotted lines indicate the criterion for hypoxia (2 mg L−1). Irradiances in depths were calculated using surface water values and extinction coefficients. 
Fig. 2The number of amplicon sequence variants, grouped into higher-ranked taxonomic groups in the surface and bottom waters during three sampling events. The red horizontal bars on the top represent the occurrence of bottom-water hypoxia, whereas the blue horizontal bar represents the non-hypoxic event. Numbers on bar graphs indicate the dissolved oxygen concentration (mg L−1) during sample collection. 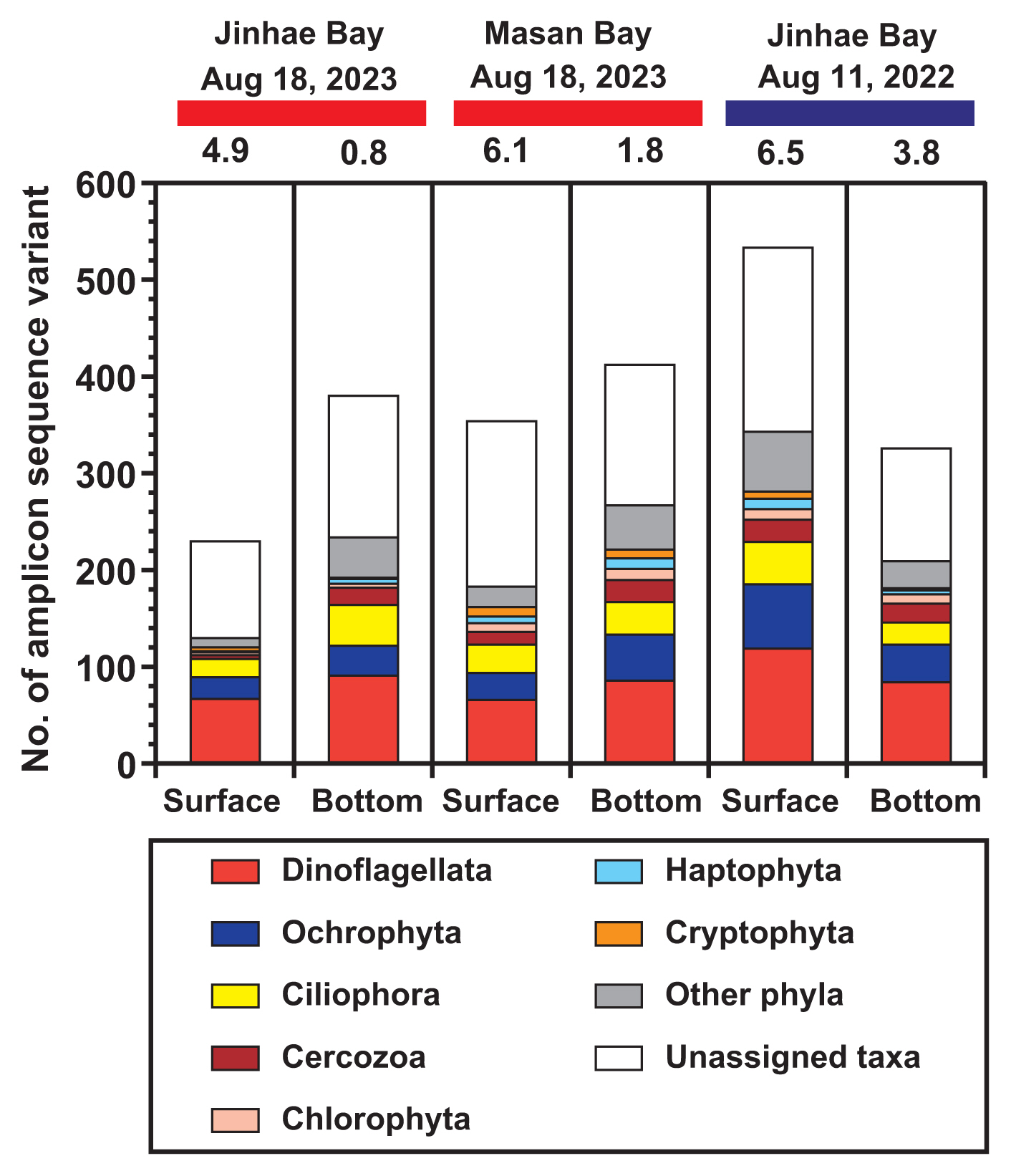
Fig. 3Venn diagrams showing the number of amplicon sequence variants (ASVs) of all protists and the dominant phyla detected in the surface water only, bottom water only, and in both the surface and bottom waters. The red horizontal bar on the top represents the occurrence of bottom-water hypoxia, whereas the blue horizontal bar represents the non-hypoxic events. 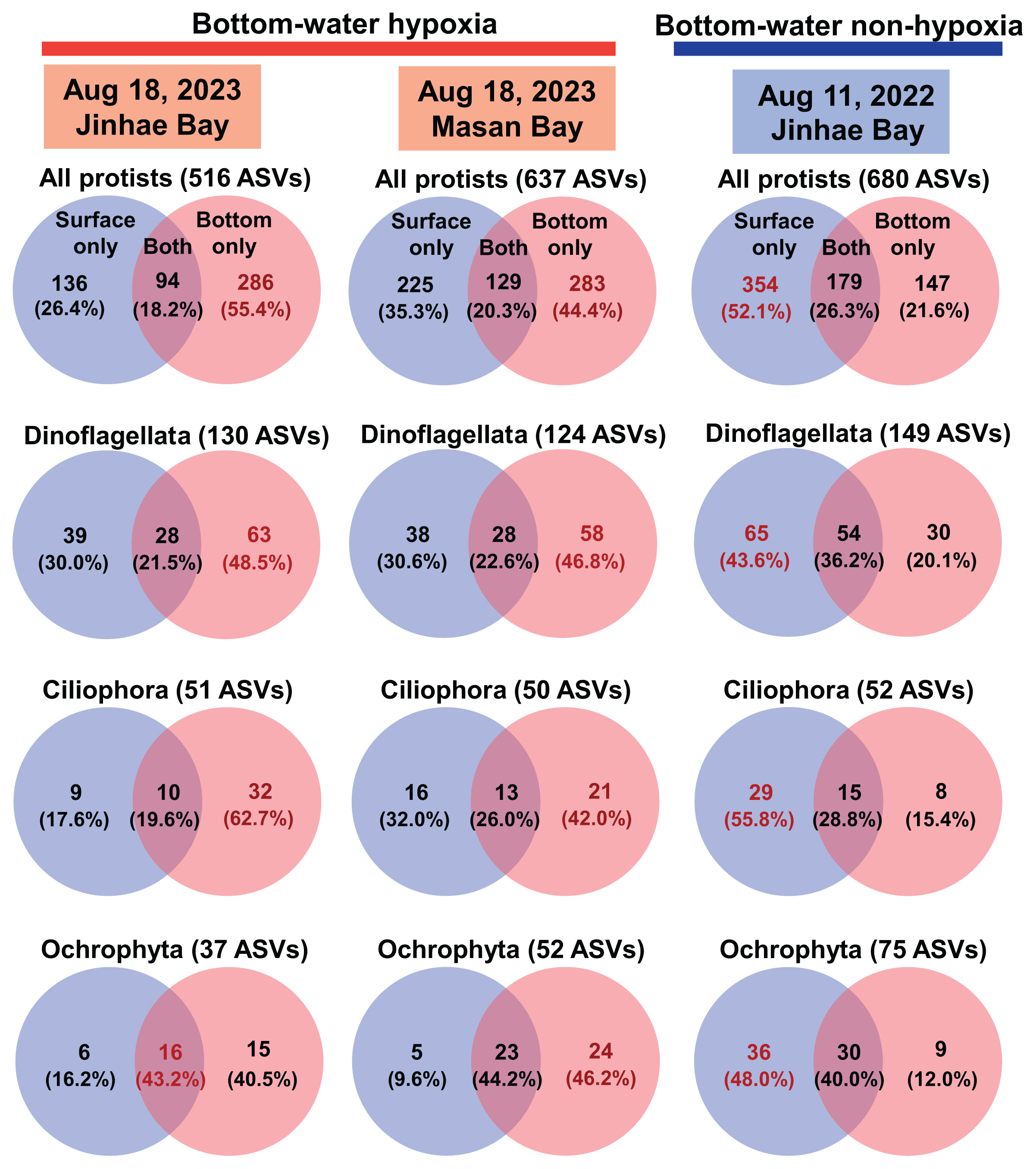
Fig. 4Relative read abundance (%) of protists based on read counts in the surface and bottom waters at three sampling events. The red horizontal bars on the top represent the occurrence of bottom-water hypoxia, whereas the blue horizontal bar represents the non-hypoxic events. Numbers on bar graphs indicate the dissolved oxygen concentration (mg L−1) during sample collection. 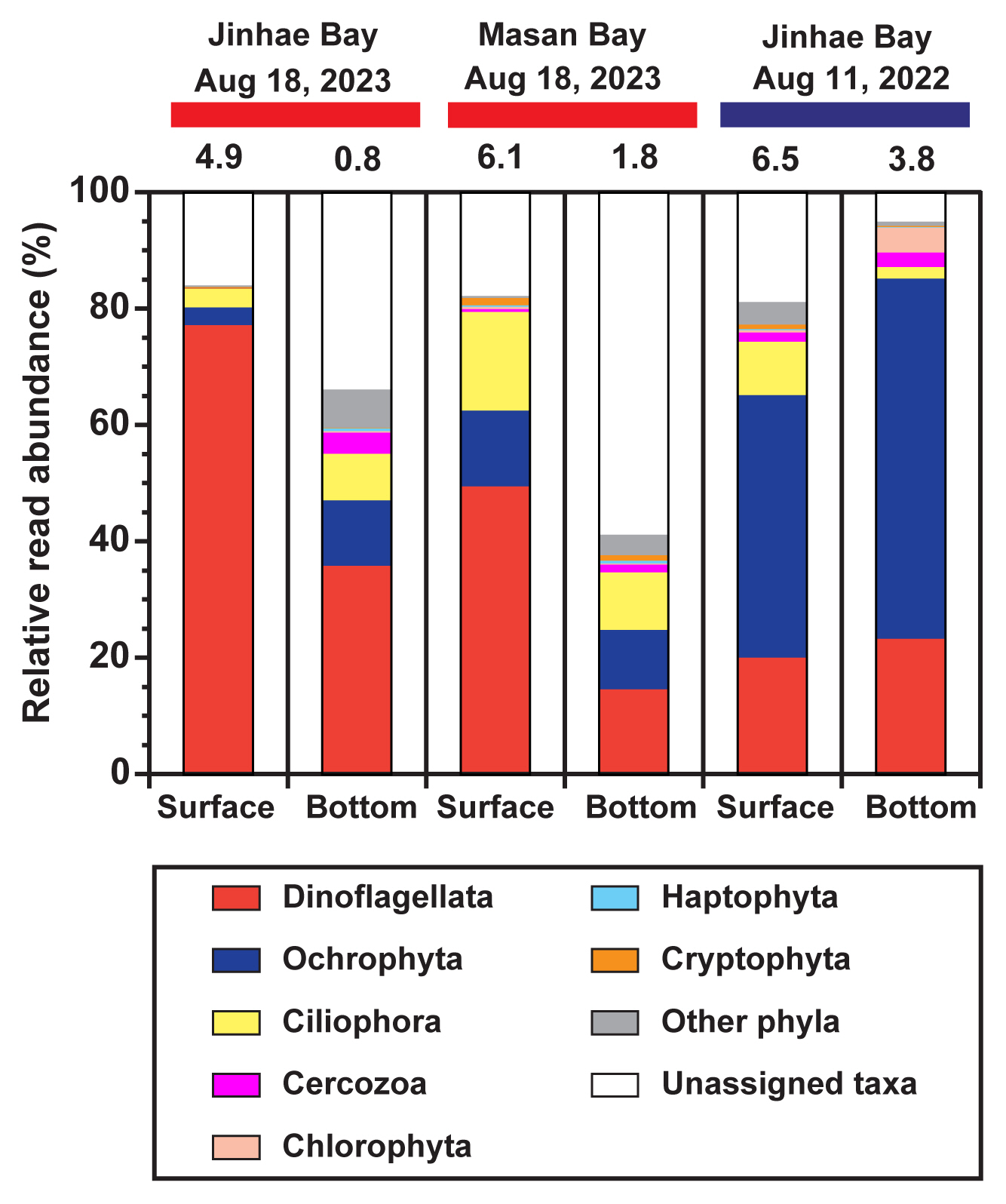
Fig. 5The number (A & B) and proportion (C & D) of amplicon sequence variants (ASVs) or species identified as autrotrophic, mixotrophic, phototrophic (whose trophic modes have not yet been explored, namely, autotrophic or mixotrophic), kleptoplastidic, and heterotrophic dinoflagellate species in the surface and bottom waters during each sampling date. Venn diagrams (E) showing the number of ASVs or species assigned as Dinoflagellata detected in the hypoxic bottom waters in Jinhae and Masan Bays. Unassigned dinoflagellate taxa were excluded from this analysis. Tables 2 and 3 provide reference information. The red horizontal bars on the top represent the occurrence of bottom-water hypoxia, whereas the blue horizontal bars represent the non-hypoxic events. Numbers on bar graphs indicate the dissolved oxygen concentration (mg L−1) during sample collection. 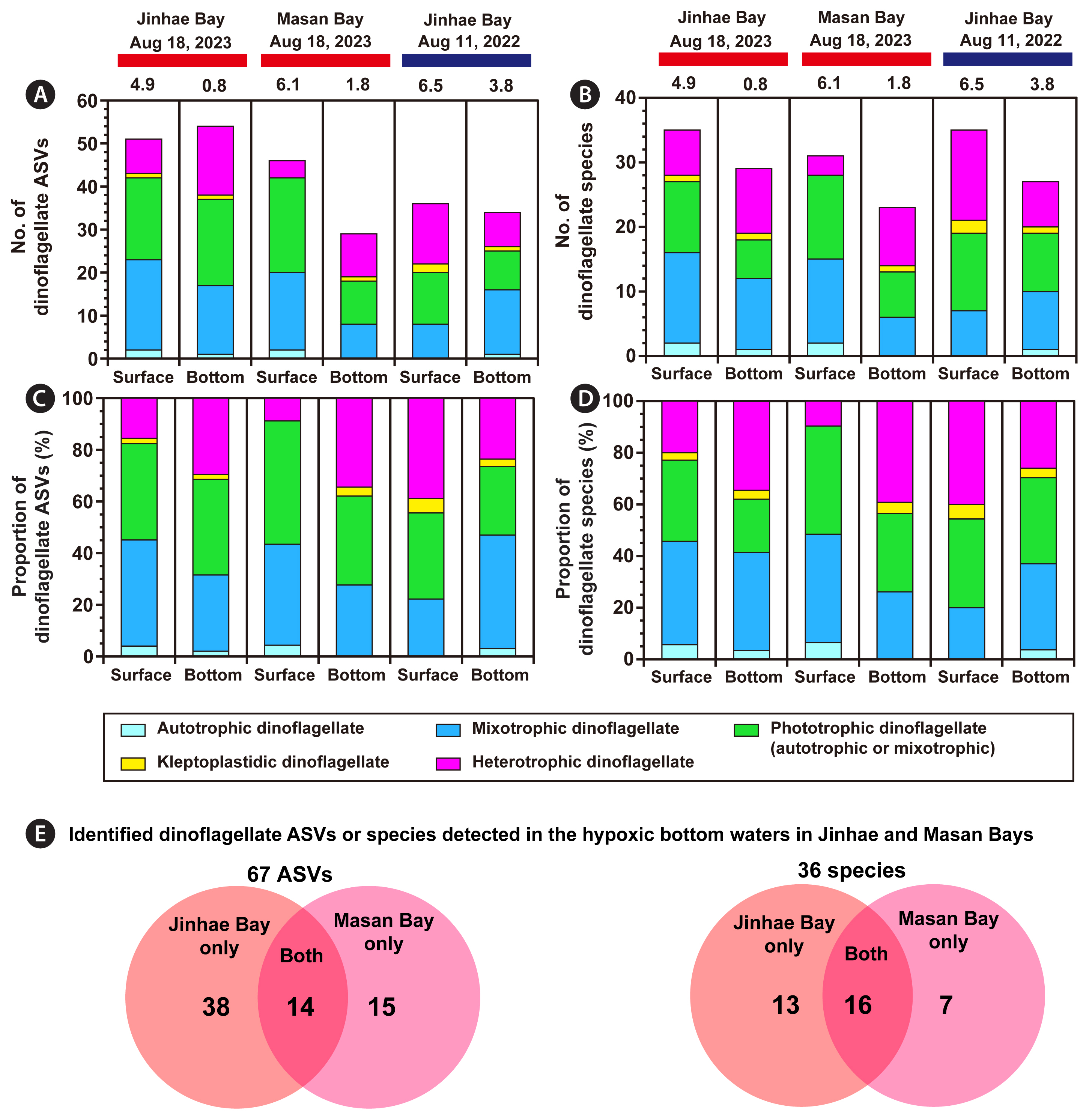
Fig. 6Bottom dissolved oxygen concentrations (DO) as a function of the surface chlorophyll-a concentration (Chl-a) during the study period. 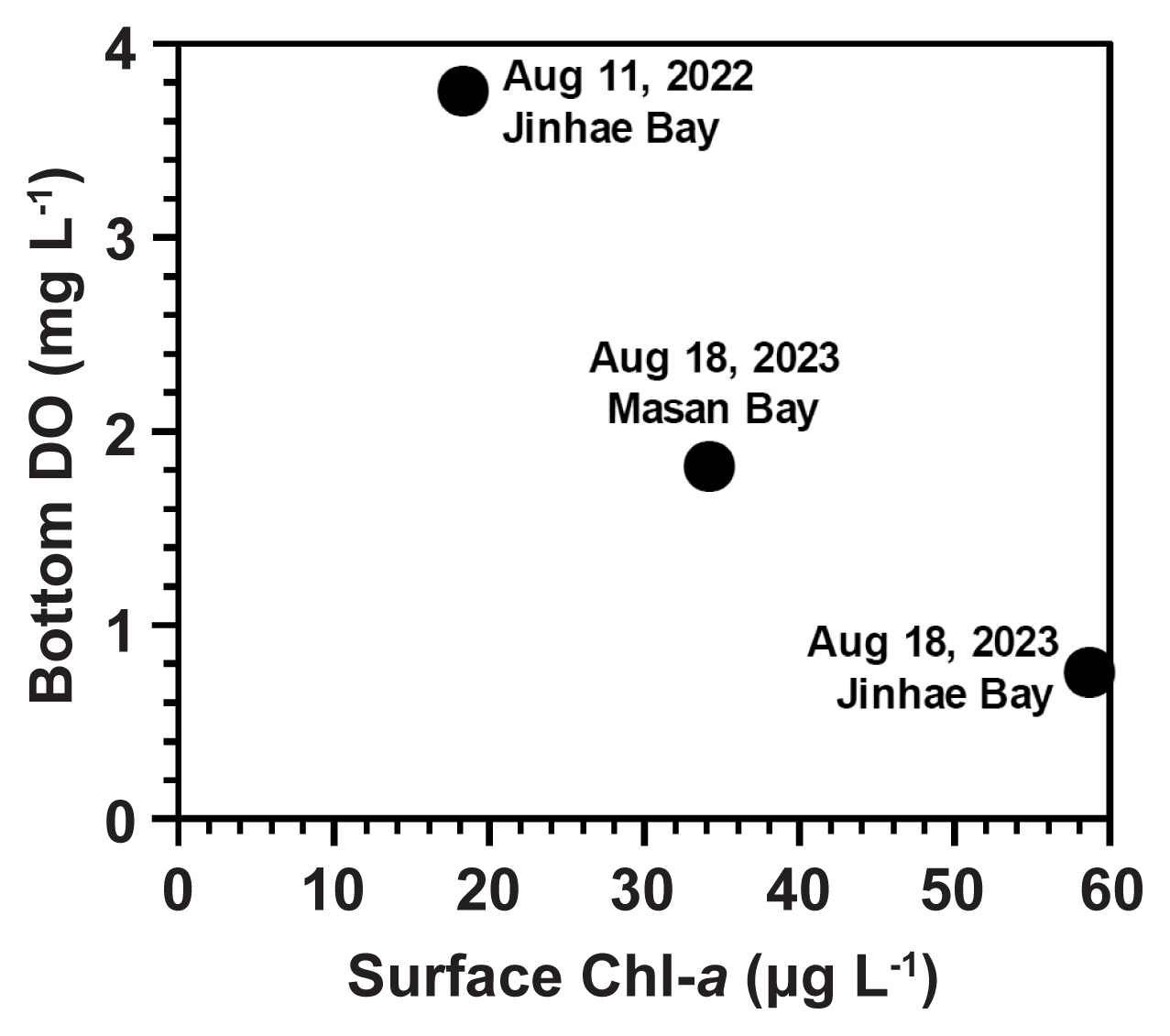
Table 1Chemical properties, including dissolved oxygen (DO) and nutrient concentrations, in the surface and bottom waters during each sample collection Table 2List of phototrophic dinoflagellates detected in each sample through metabarcoding analysis and their trophic modes (TMs)
The numbers in parentheses represent the dissolved oxygen concentrations (mg L−1) during sample collection. Boldfaces indicate dinoflagellate species detected under hypoxic conditions. The dinoflagellate species in the list were identified based on the amplicon sequence variant data. Table 3List of kleptoplastidic and heterotrophic dinoflagellates detected in each sample through metabarcoding analysis and their trophic modes (TMs)
Table 4List and the number of the dinoflagellate species surviving in hypoxic waters (<2 mg L−1) in the present study, categorized into two groups: previously known and newly discovered
Table 5List of the dinoflagellate species surviving in the hypoxic bottom waters (<2 mg L−1) in Jinhae Bay only, Masan Bay only, or both stations on Aug 18, 2023, based on metabarcoding analyses REFERENCESAzam, F., Fenchel, T., Field, J. G., Gray, J. S., Meyer-Reil, L. A. & Thingstad, F. 1983. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10:257–263.
Bendtsen, J. & Hansen, J. L. S. 2013. Effects of global warming on hypoxia in the Baltic Sea–North Sea transition zone. Ecol. Modell. 264:17–26.
Blossom, H. E., Daugbjerg, N. & Hansen, P. J. 2012. Toxic mucus traps: a novel mechanism that mediates prey uptake in the mixotrophic dinoflagellate Alexandrium pseudogonyaulax
. Harmful Algae. 17:40–53.
Bockstahler, K. R. & Coats, D. W. 1993. Grazing of the mixotrophic dinoflagellate Gymnodinium sanguineum on ciliate populations of Chesapeake Bay. Mar. Biol. 116:477–487.
Breitburg, D., Levin, L. A., Oschlies, A., Grégoire, M., Chavez, F. P., Conley, D. J., Garçon, V., Gilbert, D., Gutiérrez, D., Isensee, K., Jacinto, G. S., Limburg, K. E., Montes, I., Naqvi, S. W. A., Pitcher, G. C., Rabalais, N. N., Roman, M. R., Rose, K. A., Seibel, B. A., Telszewski, M., Yasuhara, M. & Zhang, J. 2018. Declining oxygen in the global ocean and coastal waters. Science. 359:eaam7240 pp.
Buskey, E. J. 1997. Behavioral components of feeding selectivity of the heterotrophic dinoflagellate Protoperidinium pellucidum
. Mar. Ecol. Prog. Ser. 153:77–89.
Buskey, E. J., Strom, S. & Coulter, C. 1992. Biolumiscence of heterotrophic dinoflagellates from Texas coastal waters. J. Exp. Mar. Biol. Ecol. 159:37–49.
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A. & Holmes, S. P. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 13:581–583.
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K. & Madden, T. L. 2009. BLAST+: architecture and applications. BMC Bioinform. 10:1–9.
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., Fierer, N., Peña, A. G., Goodrich, J. K., Gordon, J. I., Huttley, G. A., Kelley, S. T., Knights, D., Koenig, J. E., Ley, R. E., Lozupone, C. A., McDonald, D., Muegge, B. D., Pirrung, M., Reeder, J., Sevinsky, J. R., Turnbaugh, P. J., Walters, W. A., Widmann, J., Yatsumenko, T., Zaneveld, J. & Knight, R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7:335–336.
Caron, D. A., Countway, P. D., Jones, A. C., Kim, D. Y. & Schnetzer, A. 2012. Marine protistan diversity. Ann. Rev. Mar. Sci. 4:467–493.
Caroppo, C., Congestri, R. & Bruno, M. 1999. On the presence of Phalacroma rotundatum in the southern Adriatic Sea (Italy). Aquat. Microb. Ecol. 17:301–310.
Chen, C.-C., Shiah, F.-K., Gong, G.-C. & Chen, T.-Y. 2021. Impact of upwelling on phytoplankton blooms and hypoxia along the Chinese coast in the East China. Sea. Mar. Pollut. Bull. 167:112288 pp.
Coats, D. W. 1999. Parasitic life styles of marine dinoflagellates. J. Eukaryot. Microbiol. 46:402–409.
Coats, D. W., Bachvaroff, T. R. & Delwiche, C. F. 2012. Revision of the family Duboscquellidae with description of Euduboscquella crenulata n. gen., n. sp. (Dinoflagellata, Syndinea), an intracellular parasite of the ciliate Favella panamensis Kofoid & Campbell. J. Eukaryot. Microbiol. 59:1–11.
Coats, D. W. & Moon, E. 2022. Ultrastructure of selected life history stages of the parasitic dinoflagellate Euduboscquella cachoni
. J. Eukaryot. Microbiol. 69:e12921 pp.
Dias, A. & Kurian, S. 2022. Characterization of colored dissolved organic matter along the western continental shelf of India during the seasonal hypoxia. Estuar. Coast. Shelf Sci. 265:107714 pp.
Edgcomb, V. P. 2016. Marine protist associations and environmental impacts across trophic levels in the twilight zone and below. Curr. Opin. Microbiol. 31:169–175.
Eom, S. H., Jeong, H. J., Ok, J. H., Park, S. A., Kang, H. C. & You, J. H. 2024. Combined effects of hypoxia and starvation on the survival and growth rates of autotrophic, mixotrophic, and heterotrophic dinoflagellates. Mar. Biol. In press.
Eom, S. H., Jeong, H. J., Ok, J. H., Park, S. A., Kang, H. C., You, J. H., Lee, S. Y., Yoo, Y. D., Lim, A. S. & Lee, M. J. 2021. Interactions between common heterotrophic protists and the dinoflagellate Tripos furca: implication on the long duration of its red tides in the South Sea of Korea in 2020. Algae. 36:25–36.
Gran-Stadniczeñko, S., Egge, E., Hostyeva, V., Logares, R., Eikrem, W. & Edvardsen, B. 2019. Protist diversity and seasonal dynamics in Skagerrak plankton communities as revealed by metabarcoding and microscopy. J. Eukaryot. Microbiol. 66:494–513.
Hallegraeff, G. M. 2004. Harmful algal blooms: a global overview. In : Hallegraeff G. M., editor In : Anderson D. M., Cembella A. D., editors Manual on Harmful Marine Microalgae. UNESCO, Paris, 25–49.
Hansen, P. J. 1991.
Dinophysis: a planktonic dinoflagellate genus which can act both as a prey and a predator of a ciliate. Mar. Ecol. Prog. Ser. 69:201–204.
Hansen, P. J. 1992. Prey size selection, feeding rates and growth dynamics of heterotrophic dinoflagellates with special emphasis on Gyrodinium spirale
. Mar. Biol. 114:327–334.
Hoppenrath, M. 2000. Morphology and taxonomy of Sinophysis (Dinophyceae, Dinophysiales) including two new marine sand-dwelling species from the North German Wadden Sea. Eur. J. Phycol. 35:153–162.
Jacobson, D. M. & Anderson, D. M. 1996. Widespread phagocytosis of ciliates and other protists by marine mixotrophic and heterotrophic thecate dinoflagellates. J. Phycol. 32:279–285.
Jang, S. H., Jeong, H. J., Kwon, J. E. & Lee, K. H. 2017. Mixotrophy in the newly described dinoflagellate Yihiella yeosuensis: a small, fast dinoflagellate predator that grows mixotrophically, but not autotrophically. Harmful Algae. 62:94–103.
Jang, S. H., Lim, P., Torano, O., Neave, E. F., Seim, H. & Marchetti, A. 2022. Protistan communities within the Galapagos archipelago with an emphasis on micrograzers. Front. Mar. Sci. 9:811979.
Jeong, H. J. 1994. Predation by the heterotrophic dinoflagellate Protoperidinium cf. divergens on copepod eggs and early naupliar stages. Mar. Ecol. Prog. Ser. 114:203–208.
Jeong, H. J. 1999. The ecological roles of heterotrophic dinoflagellates in marine planktonic community. J. Eukaryot. Microbiol. 46:390–396.
Jeong, H. J., Kang, H. C., Lim, A. S., Jang, S. H., Lee, K., Lee, S. Y., Ok, J. H., You, J. H., Kim, J. H., Lee, K. H., Park, S. A., Eom, S. H., Yoo, Y. D. & Kim, K.Y. 2021. Feeding diverse prey as an excellent strategy of mixotrophic dinoflagellates for global dominance. Sci. Adv. 7:eabe4214 pp.
Jeong, H. J., Kim, J. S., Kim, J. H., Kim, S. T., Seong, K. A., Kim, T. H., Song, J. Y. & Kim, S. K. 2005a. Feeding and grazing impact of the newly described heterotrophic dinoflagellate Stoeckeria algicida on the harmful alga Heterosigma akashiwo
. Mar. Ecol. Prog. Ser. 295:69–78.
Jeong, H. J., Kim, J. S., Yoo, Y. D., Kim, S. T., Kim, T. H., Park, M. G., Lee, C. H., Seong, K. A., Kang, N. S. & Shim, J. H. 2003. Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: a potential biological method to control red tides using mass-cultured grazers. J. Eukaryot. Microbiol. 50:274–282.
Jeong, H. J., Kim, S. K., Kim, J. S., Kim, S. T., Yoo, Y. D. & Yoon, J. Y. 2001. Growth and grazing rates of the heterotrophic dinoflagellate Polykrikos kofoidii on red-tide and toxic dinoflagellates. J. Eukaryot. Microbiol. 48:298–308.
Jeong, H. J., Lee, K. H., Yoo, Y. D., Kang, N. S. & Lee, K. 2011. Feeding by the newly described, nematocyst-bearing heterotrophic dinoflagellate Gyrodiniellum shiwhaense
. J. Eukaryot. Microbiol. 58:511–524.
Jeong, H. J., Lim, A. S., Franks, P. J. S., Lee, K. H., Kim, J. H., Kang, N. S., Lee, M. J., Jang, S. H., Lee, S. Y., Yoon, E. Y., Park, J. Y., Yoo, Y. D., Seong, K. A., Kwon, J. E. & Jang, T. Y. 2015. A hierarchy of conceptual models of red-tide generation: nutrition, behavior, and biological interactions. Harmful Algae. 47:97–115.
Jeong, H. J., Park, J. Y., Nho, J. H., Park, M. O., Ha, J. H., Seong, K. A., Jeng, C., Seong, C. N., Lee, K. Y. & Yih, W. H. 2005b. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus
. Aquat. Microb. Ecol. 41:131–143.
Jeong, H. J., Seong, K. A., Yoo, Y. D., Kim, T. H., Kang, N. S., Kim, S., Park, J. Y., Kim, J. S., Kim, G. H. & Song, J. Y. 2008. Feeding and grazing impact by small marine heterotrophic dinoflagellates on heterotrophic bacteria. J. Eukaryot. Microbiol. 55:271–288.
Jeong, H. J., Yoo, Y. D., Kim, J. S., Seong, K. A., Kang, N. S. & Kim, T. H. 2010. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci. J. 45:65–91.
Jeong, H. J., Yoo, Y. D., Kim, S. T. & Kang, N. S. 2004. Feeding by the heterotrophic dinoflagellate Protoperidinium bipes on the diatom Skeletonema costatum
. Aquat. Microb. Ecol. 36:171–179.
Jeong, H. J., Yoo, Y. D., Lee, K. H., Kim, T. H., Seong, K. A., Kang, N. S., Lee, S. Y., Kim, J. S., Kim, S. & Yih, W. H. 2013. Red tides in Masan Bay, Korea in 2004–2005: I. Daily variations in the abundance of red-tide organisms and environmental factors. Harmful Algae. 30:S75–S88.
Jeong, H. J., Yoo, Y. D., Park, J. Y., Song, J. Y., Kim, S. T., Lee, S. H., Kim, K. Y. & Yih, W. H. 2005c. Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. Aquat. Microb. Ecol. 40:133–150.
Jeong, H. J., Yoo, Y. D., Seong, K. A., Kim, J. H., Park, J. Y., Kim, S., Lee, S. H., Ha, J. H. & Yih, W. H. 2005d. Feeding by the mixotrophic red-tide dinoflagellate Gonyaulax polygramma: mechanisms, prey species, effects of prey concentration, and grazing impact. Aquat. Microb. Ecol. 38:249–257.
Kang, H. C., Jeong, H. J., Lim, A. S., Ok, J. H., You, J. H., Park, S. A. & Eom, S. H. 2023. Feeding by common heterotrophic protists on the mixotrophic dinoflagellate Ansanella granifera (Suessiaceae, Dinophyceae). Algae. 38:57–70.
Kang, H. C., Jeong, H. J., Park, S. A., Eom, S. H., Ok, J. H., You, J. H., Jang, S. H. & Lee, S. Y. 2020. Feeding by the newly described heterotrophic dinoflagellate Gyrodinium jinhaense: comparison with G. dominans and G. moestrupii
. Mar. Biol. 167:156 pp.
Kawami, H., Van Wezel, R., Koeman, R. P. T. & Matsuoka, K. 2009.
Protoperidinium tricingulatum sp. nov. (Dinophyceae), a new motile form of a round, brown, and spiny dinoflagellate cyst. Phycol. Res. 57:259–267.
Kemp, W. M., Testa, J. M., Conley, D. J., Gilbert, D. & Hagy, J. D. 2009. Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences. 6:2985–3008.
Kim, J. S. & Jeong, H. J. 2004. Feeding by the heterotrophic dinoflagellates Gyrodinium dominans and G. spirale on the red-tide dinoflagellate Prorocentrum minimum
. Mar. Ecol. Prog. Ser. 280:85–94.
Kim, S. & Park, M. G. 2017. Feeding characteristics and molecular phylogeny of the thecate mixotrophic dinoflagellate Fragilidium mexicanum
. Harmful Algae. 63:154–163.
Kim, Y. H., Seo, H. J., Yang, H. J., Lee, M.-Y., Kim, T.-H., Yoo, D., Choi, B.-J. & Jang, S. H. 2023. Protistan community structure and the influence of a branch of Kuroshio in the northeastern East China Sea during the late spring. Front. Mar. Sci. 10:1192529 pp.
Kiϕrboe, T. & Titelman, J. 1998. Feeding, prey selection and prey encounter mechanisms in the heterotrophic dinoflagellate Noctiluca scintillans
. J. Plankton Res. 20:1615–1636.
Lee, J., Park, K.-T., Lim, J.-H., Yoon, J.-E. & Kim, I.-N. 2018. Hypoxia in Korean coastal waters: a case study of the natural Jinhae Bay and artificial Shihwa Bay. Front. Mar. Sci. 5:70 pp.
Lee, K. H., Jeong, H. J., Kwon, J. E., Kang, H. C., Kim, J. H., Jang, S. H., Park, J. Y., Yoon, E. Y. & Kim, J. S. 2016. Mixotrophic ability of the phototrophic dinoflagellates Alexandrium andersonii, A. affine, and A. fraterculus
. Harmful Algae. 59:67–81.
Lee, M. J., Jeong, H. J., Lee, K. H., Jang, S. H., Kim, J. H. & Kim, K. Y. 2015. Mixotrophy in the nematocyst-taeniocyst complex-bearing phototrophic dinoflagellate Polykrikos hartmannii
. Harmful Algae. 49:124–134.
Lee, S. K., Jeong, H. J., Jang, S. H., Lee, K. H., Kang, N. S., Lee, M. J. & Potvin, É. 2014. Mixotrophy in the newly described dinoflagellate Ansanella granifera: feeding mechanism, prey species, and effect of prey concentration. Algae. 29:137–152.
Lee, S. Y., Jeong, H. J., Kang, H. C., Ok, J. H., You, J. H., Park, S. A. & Eom, S. H. 2021. Comparison of the spatial-temporal distributions of the heterotrophic dinoflagellates Gyrodinium dominans, G. jinhaense, and G. moestrupii in Korean coastal waters. Algae. 36:37–50.
Lee, S. Y., Jeong, H. J., Ok, J. H., Kang, H. C. & You, J. H. 2020. Spatial-temporal distributions of the newly described mixotrophic dinoflagellate Gymnodinium smaydae in Korean coastal waters. Algae. 35:225–236.
Lewitus, A. J., Glasgow, H. B. Jr & Burkholder, J. M. 1999. Kleptoplastidy in the toxic dinoflagellate Pfiesteria piscicida (Dinophyceae). J. Phycol. 35:303–312.
Li, A., Stoecker, D. K. & Adolf, J. E. 1999. Feeding, pigmentation, photosynthesis and growth of the mixotrophic dinoflagellate Gyrodinium galatheanum
. Aquat. Microb. Ecol. 19:163–176.
Lim, H.-S., Diaz, R. J., Hong, J.-S. & Schaffner, L. C. 2006. Hypoxia and benthic community recovery in Korean coastal waters. Mar. Pollut. Bull. 52:1517–1526.
Martin, M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17:10–12.
Martinez, E., Vélez, S. M., Mayo, M. & Sastre, M. P. 2016. Acute toxicity assessment of N,N-diethyl-m-toluamide (DEET) on the oxygen flux of the dinoflagellate Gymnodinium instriatum
. Ecotoxicology. 25:248–252.
Massana, R. 2015. Protistan diversity in environmental molecular surveys. In : Ohtsuka S., Suzaki T., Horiguchi T., Suzuki N., Not F., editors Marine Protists: Diversity and Dynamics. Springer, Tokyo, 3–21.
Min, J. & Kim, K. Y. 2023. Diversity and assembly of planktonic protist communities in the Jeju Strait, Korea. Front. Mar. Sci. 10:1225640 pp.
Mitra, A., Flynn, K. J., Burkholder, J. M., Berge, T., Calbet, A., Raven, J. A., Granéli, E., Glibert, P. M., Hansen, P. K., Stoecker, D. K., Thingstad, F., Tillmann, U., Våge, S., Wilken, S. & Zubkov, M. V. 2014. The role of mixotrophic protists in the biological carbon pump. Biogeosciences. 11:995–1005.
Moestrup, Ø., Lindberg, K. & Daugbjerg, N. 2009. Studies on woloszynskioid dinoflagellates V. Ultrastructure of Biecheleriopsis gen. nov., with description of Biecheleriopsis adriatica sp. nov. Phycol. Res. 57:221–237.
Nakamura, Y. & Hirata, A. 2006. Plankton community structure and trophic interactions in a shallow and eutrophic estuarine system, Ariake Sound, Japan. Aquat. Microb. Ecol. 44:45–57.
Nilsson, H. C. & Rosenberg, R. 1994. Hypoxic response of two marine benthic communities. Mar. Ecol. Prog. Ser. 115:209–217.
Ohtsuka, S., Suzaki, T., Horiguchi, T., Suzuki, N. & Not, F. 2015. Marine protists: diversity and dynamics. Springer, Tokyo, 637 pp.
Ok, J. H., Jeong, H. J., Kang, H. C., Park, S. A., Eom, S. H., You, J. H. & Lee, S. Y. 2021a. Ecophysiology of the kleptoplastidic dinoflagellate Shimiella gracilenta: I. spatiotemporal distribution in Korean coastal waters and growth and ingestion rates. Algae. 36:263–283.
Ok, J. H., Jeong, H. J., Kang, H. C., Park, S. A., Eom, S. H., You, J. H. & Lee, S. Y. 2022. Ecophysiology of the kleptoplastidic dinoflagellate Shimiella gracilenta: II. Effects of temperature and global warming. Algae. 37:49–62.
Ok, J. H., Jeong, H. J., Lim, A. S., Kang, H. C., You, J. H., Park, S. A. & Eom, S. H. 2023a. Lack of mixotrophy in three Karenia species and the prey spectrum of Karenia mikimotoi (Gymnodiniales, Dinophyceae). Algae. 38:39–55.
Ok, J. H., Jeong, H. J., Lim, A. S., Lee, S. Y. & Kim, S. J. 2018. Feeding by the heterotrophic nanoflagellate Katablepharis remigera on algal prey and its nationwide distribution in Korea. Harmful Algae. 74:30–45.
Ok, J. H., Jeong, H. J., Lim, A. S., You, J. H., Yoo, Y. D., Kang, H. C., Park, S. A., Lee, M. J. & Eom, S. H. 2023b. Effects of intrusion and retreat of deep cold waters on the causative species of red tides offshore in the South Sea of Korea. Mar. Biol. 170:6 pp.
Ok, J. H., Jeong, H. J., You, J. H., Kang, H. C., Park, S. A., Lim, A. S., Lee, S. Y. & Eom, S. H. 2021b. Phytoplankton bloom dynamics in incubated natural seawater: predicting bloom magnitude and timing. Front. Mar. Sci. 8:681252 pp.
Piredda, R., Tomasino, M. P., D’Erchia, A. M., Manzari, C., Pesole, G., Montresor, M., Kooistra, W. H. C. F., Sarno, D. & Zingone, A. 2017. Diversity and temporal patterns of planktonic protist assemblages at a Mediterranean Long Term Ecological Research site. FEMS Microbiol. Ecol. 93:fiw200 pp.
Rabalais, N. N., Turner, R. E., Díaz, R. J. & Justić, D. 2009. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 66:1528–1537.
Rajan, A., Thankamony, R., Othman, Y., Khan, S. A. & Jamali, E. A. 2021. Massive bloom of Cochlodinium polykrikoides and its impacts in the United Arab Emirates’ waters. Int. J. Ecol. Environ. Sci. 3:341–347.
Ratmaya, W., Laverman, A. M., Rabouille, C., Akbarzadeh, Z., Andrieux-Loyer, F., Barillé, L., Barillé, A.-L., Merrer, Y. L. & Souchu, P. 2022. Temporal and spatial variations in benthic nitrogen cycling in a temperate macro-tidal coastal ecosystem: observation and modeling. Cont. Shelf Res. 235:104649 pp.
Reñé, A., Camp, J. & Garcés, E. 2014.
Polykrikos tanit sp. nov., a new mixotrophic unarmoured pseudocolonial dinoflagellate from the NW Mediterranean Sea. Protist. 165:81–92.
Riedel, B., Zuschin, M. & Stachowitsch, M. 2012. Tolerance of benthic macrofauna to hypoxia and anoxia in shallow coastal seas: a realistic scenario. Mar. Ecol. Prog. Ser. 458:39–52.
Rocke, E., Jing, H. & Liu, H. 2013. Phylogenetic composition and distribution of picoeukaryotes in the hypoxic northwestern coast of the Gulf of Mexico. Microbiologyopen. 2:130–143.
Rocke, E., Jing, H., Xia, X. & Liu, H. 2016. Effects of hypoxia on the phylogenetic composition and species distribution of protists in a subtropical harbor. Microb. Ecol. 72:96–105.
Rodríguez, F., Escalera, L., Reguera, B., Rial, P., Riobó, P. & da Silva, T. D. J. 2012. Morphological variability, toxinology and genetics of the dinoflagellate Dinophysis tripos (Dinophysiaceae, Dinophysiales). Harmful Algae. 13:26–33.
Sakamoto, S., Lim, W. A., Lu, D., Dai, X., Orlova, T. & Iwataki, M. 2021. Harmful algal blooms and associated fisheries damage in East Asia: current status and trends in China, Japan, Korea and Russia. Harmful Algae. 102:101787 pp.
Saldarriaga, J. F., Leander, B. S., “Max” Taylor, F. J. R. & Keeling, P. J. 2003.
Lessardia elongata gen. et sp. nov. (Dinoflagellata, Peridiniales, Podolampaceae) and the taxonomic position of the genus Roscoffia
. J. Phycol. 39:368–378.
Sampaio, E., Santos, C., Rosa, I. C., Ferreira, V., Pörtner, H.-O., Duarte, C. M., Levin, L. A. & Rosa, R. 2021. Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 5:311–321.
Sampedro, N., Fraga, S., Penna, A., Casabianca, S., Zapata, M., Grünewald, C. F., Riobó, P. & Camp, J. 2011.
Barrufeta bravensis gen. nov. sp. nov. (Dinophyceae): a new bloom-forming species from the Northwest Mediterranean sea. J. Phycol. 47:375–392.
Santoferrara, L., Burki, F., Filker, S., Logares, R., Dunthorn, M. & McManus, G. B. 2020. Perspectives from ten years of protist studies by high-throughput metabarcoding. J. Eukaryot. Microbiol. 67:612–622.
Santoferrara, L. F., McManus, G. B., Greenfield, D. I. & Smith, S. A. 2022. Microbial communities (bacteria, archaea and eukaryotes) in a temperate estuary during seasonal hypoxia. Aquat. Microb. Ecol. 88:61–79.
Schmidtko, S., Stramma, L. & Visbeck, M. 2017. Decline in global oceanic oxygen content during the past five decades. Nature. 542:335–339.
Schnepf, E. & Elbrächter, M. 1992. Nutritional strategies in dinoflagellates: a review with emphasis on cell biological aspects. Eur. J. Protistol. 28:3–24.
Scully, M. E., Geyer, W. R., Borkman, D., Pugh, T. L., Costa, A. & Nichols, O. C. 2022. Unprecedented summer hypoxia in southern Cape Cod Bay: an ecological response to regional climate change? Biogeosciences. 19:3523–3536.
Sherr, E. B. & Sherr, B. F. 2007. Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser. 352:187–197.
Sinkko, H., Lukkari, K., Sihvonen, L. M., Sivonen, K., Leivuori, M., Rantanen, M., Paulin, L. & Lyra, C. 2013. Bacteria contribute to sediment nutrient release and reflect progressed eutrophication-driven hypoxia in an organic-rich continental sea. PLoS ONE. 8:e67061 pp.
Smalley, G. W. & Coats, D. W. 2002. Ecology of the red-tide dinoflagellate Ceratium furca: distribution, mixotrophy, and grazing impact on ciliate populations of Chesapeake Bay. J. Eukaryot. Microbiol. 49:63–73.
Smayda, T. J. 1997. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 42:1137–1153.
Stat, M., Morris, E. & Gates, R. D. 2008. Functional diversity in coral–dinoflagellate symbiosis. Proc. Natl. Acad. Sci. U. S. A. 105:9256–9261.
Stauffer, B. A., Schnetzer, A., Gellene, A. G., Oberg, C., Sukhatme, G. S. & Caron, D. A. 2013. Effects of an acute hypoxic event on microplankton community structure in a coastal harbor of Southern California. Estuar Coasts. 36:135–148.
Stoeck, T., Bass, D., Nebel, M., Christen, R., Jones, M. D. M., Breiner, H.-W. & Richards, T. A. 2010. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 19:21–31.
Stoecker, D. K., Hansen, P. J., Caron, D. A. & Mitra, A. 2017. Mixotrophy in the marine plankton. Annu. Rev. Mar. Sci. 9:311–335.
Strom, S. L. & Buskey, E. J. 1993. Feeding, growth, and behavior of the thecate heterotrophic dinoflagellate Oblea rotunda
. Limnol. Oceanogr. 38:965–977.
Takano, Y. & Horiguchi, T. 2004. Surface ultrastructure and molecular phylogenetics of four unarmored heterotrophic dinoflagellates, including the type species of the genus Gyrodinium (Dinophyceae). Phycol. Res. 52:107–116.
Telesh, I., Schubert, H. & Skarlato, S. 2013. Life in the salinity gradient: discovering mechanisms behind a new biodiversity pattern. Estuar. Coast. Shelf Sci. 135:317–327.
Thimijan, R. W. & Heins, R. D. 1983. Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. HortScience. 18:818–822.
Yamaguchi, A., Hoppenrath, M., Pospelova, V., Horiguchi, T. & Leander, B. S. 2011. Molecular phylogeny of the marine sand-dwelling dinoflagellate Herdmania litoralis and an emended description of the closely related planktonic genus Archaeperidinium Jörgensen. Eur. J. Phycol. 46:98–112.
Yoo, Y. D., Jeong, H. J., Kang, N. S., Song, J. Y., Kim, K. Y., Lee, G. & Kim, J. 2010. Feeding by the newly described mixotrophic dinoflagellate Paragymnodinium shiwhaense: feeding mechanism, prey species, and effect of prey concentration. J. Eukaryot. Microbiol. 57:145–158.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||