Plastid-associated galactolipid composition in eyespot-containing dinoflagellates: a review
Article information
Abstract
Relative to the large number of photosynthetic dinoflagellate species, only a select few possess proteinaceous, carotenoid-rich eyespots which have been demonstrated in other algae to act in phototactic responses. The proteins comprising the different categories of dinoflagellate eyespots are positioned in or near the peridinin-containing photosynthetic plastid membranes which are composed primarily of two galactolipids, mono- and digalactosyldiacylglycerol (MGDG and DGDG). Within eyespot-containing dinoflagellates, this arrangement occurs mostly in those with secondary plastids, although some dinoflagellates with tertiary plastids of diatom origin are known to possess eyespots. We here provide an examination of the MGDG and DGDG composition of eyespot-containing dinoflagellates with secondary, peridinin-containing plastids and tertiary plastids of diatom origin to address the fundamental question of whether eyespots and their component proteins and carotenoids are associated with alterations in galactolipid composition when compared to eyespot-lacking photosynthetic dinoflagellates. This is an important question because the dinoflagellate eyespot-plastid membrane system can be considered a more complicated and evolved state of plastid development. Included in this examination are data on the previously unexamined peridinin- and type A eyespot-containing dinoflagellate Margalefidinium polykrikoides, and the type D eyespot-containing, aberrant plastid “dinotom” Durinskia baltica. In addition, we have reviewed the galactolipid composition of algae from the Chlorophyceae, Cryptophyceae, and Euglenophyceae as a comparison to determine if algal classes apart from the Dinophyceae contain altered galactolipids in association with eyespots. We conclude that the presence of an eyespot in dinoflagellates and other algae is not associated with noticeable changes in galactolipid composition.
INTRODUCTION
Within eukaryotic algae, there are a number of taxa which possess eyespots (Dodge 1974), where it is thought that the eyespot acts as a light sensor to direct flagellar movement to propel cells towards or away from a light source (Foster and Smyth 1980). The eyespot is often associated in some manner with the chloroplast, which is typical of eyespot-containing members of the classes Chlorophyceae, Chrysophyceae, and some algae within the Cryptophyceae (Dodge 1974). However, the eyespot can be separate from the chloroplast, as seen in the class Euglenophyceae (Dodge 1974). A number of photosynthetic dinoflagellate taxa have also been observed to possess eyespots, with at least six types of eyespots (types A–F described below) observed, as based on their degree of association with the chloroplast. Types A–C describe the eyespots in various algae including dinoflagellates; however, types D–F describe eyespots found only in dinoflagellates (Dodge 1974, Moestrup and Daugbjerg 2007, Craveiro et al. 2010).
Regardless of their association with chloroplasts, all eyespots contain carotenoid pigments which can vary in terms of arrangement within the eyespot (Dodge 1974). The eyespots of Euglenophyceae contain a single layer of different-sized pigment granules that are not uniformly spaced within the eyespot membrane (Walne and Arnott 1967, Kato et al. 2020). Eyespots of Chlorophyceae, including the model green alga Chlamydomonas reinhardtii P. A. Dangeard, typically contain a single or multiple layer(s) of pigments in which the pigments are hexagonally arranged between the inner chloroplast membrane and the chloroplast lamellae (Nakamura et al. 1973, Foster and Smyth 1980). While classes of algae other than dinoflagellates tend to be characterized by the presence of the same type of eyespot within a particular algal class, the Dinophyceae are characterized by multiple types of eyespots, and as such, dinoflagellate eyespot pigment arrangement also varies (Kreimer 1999 and references therein).
Eyespots located within, or in contact with, plastid membranes will have association with the galactolipids which comprise those membranes. Photosynthetic algae rely on the galactolipids mono- and digalactosyldiacylglycerol (MGDG and DGDG, respectively) as prominent components of thylakoid membranes within chloroplasts where photosynthesis occurs (Boudière et al. 2014). Within the thylakoid membrane, MGDG and DGDG constitute the lipid matrix for the photosystems which are the photosynthetic functional units containing light-harvesting complexes (Allen 2002, Loll et al. 2005, Gao et al. 2018). The structures of MGDG and DGDG differ in which MGDG does not form bilayers while DGDG does, so the abundance and arrangement of each galactolipid is important for thylakoid membrane functionality (Murphy 1982, Demé et al. 2014). Within photosystem II, DGDG binds to stabilizing proteins to provide structural support for the photosystems (Sakurai et al. 2007). DGDG-deficient cells possess curved thylakoid membranes instead of the typical flattened membranes which results in a separation of the stroma from the thylakoid membrane system, thus highlighting the structural importance of DGDG (Dörmann et al. 1995, Hölzl et al. 2009). In addition, low MGDG levels can cause decreased numbers of thylakoid membranes and reduced sizes and altered shapes of chloroplasts (Jarvis et al. 2000, Wu et al. 2013). Therefore, due to the close associations between MGDG, DGDG and the photosystems, galactolipids have a crucial role in supporting photosystems and photosynthetic processes.
Although much is known about the MGDG and DGDG compositions of the algal classes mentioned above, there are always open questions deserving of further study. For example, does the presence of an eyespot, either within or closely associated with the chloroplast of an alga, correlate with any difference in galactolipid composition between that alga and another member of its genus which does not possess an eyespot? In other words, is the galactolipid composition of the eyespot-containing alga altered to accommodate the eyespot itself, or do changes in galactolipid composition promote eyespot formation? More specifically, because most of the categories of dinoflagellate eyespots (types A, B, D, F, and G; described below) involve some sort of interaction with plastid membranes, the question asked in this paper is: does an individual eyespot-containing dinoflagellate species have a unique galactolipid composition, such that it differs from non-eyespot-forming yet phylogenetically related species (most likely within the same MGDG and DGDG cluster, described below)? This is an important question because it will indicate whether placement of the different eyespot types within or near the chloroplast membranes is accompanied by unique MGDG and DGDG compositions. To this end, we present a reassessment of past galactolipid characterization studies with special consideration of secondary plastid, peridinin-containing dinoflagellates with eyespot types A–C and E.
The purpose of this commentary is to examine published work on the galactolipids of eyespot-containing photosynthetic dinoflagellates to answer this question. Work from our lab has shown that peridinin-containing photosynthetic dinoflagellates cluster into two groups depending on which fatty acids are associated with MGDG and DGDG (described below). Given that most of these eyespot-containing dinoflagellates possess the pigment peridinin, there are ample data on dinoflagellate galactolipids to make a literature-based comparison of eyespot-containing vs. eyespot-lacking taxa. Considering that the presence of an eyespot in or near the chloroplast represents a more evolved functionality beyond the reactions normally associated with photosynthesis, it is reasonable to hypothesize that the placement of an eyespot within, or associated with, a dinoflagellate chloroplast will correlate with a noticeable change in galactolipid composition, when compared to closely related, eyespot-lacking dinoflagellates, possibly to accommodate the positioning of the eyespot (and its associated carotenoid pigments and proteins). In addition, comparisons between members of the Dinophyceae provide a means to efficiently test this hypothesis because of the wide range of eyespot types identified among dinoflagellates, compared to members of other algal classes. Furthermore, due to the varying arrangements of eyespots in relation to the chloroplast, it is reasonable to hypothesize a correlation between eyespots and changes in galactolipids that comprise plastid membranes to either accommodate eyespots or facilitate their development, given the structural role of galactolipids as discussed above.
To this end, this review begins with an overview of the general biology of eyespots in algae other than dinoflagellates, and a discussion of the galactolipids of these algae, because their eyespot arrangement provides a useful reference for better understanding dinoflagellate eyespots. The review then provides a description of the eyespot types in dinoflagellates, and is followed by discussion on the galactolipids of these eyespot-containing dinoflagellates.
GENERAL EYESPOT BIOLOGY
C. reinhardtii has been heavily studied as an eyespot model system in which the eyespot, located in the chloroplast, detects light and induces electrical currents that modulate flagellar movement to prompt phototactic behaviors (Ueki et al. 2016). In order to detect light, carotenoid pigments in the chloroplast membrane reflect light onto or shield light from photoreceptor proteins, such as channelrhodopsins, in the plasma membrane surrounding the carotenoid pigments (Melkonian and Robenek 1984, Ueki et al. 2016). Since the eyespot is asymmetrically placed within the cell, light reaching the eyespot can originate from the same side or the opposite side of the cell in which the eyespot is located (Foster and Smyth 1980). Light entering from the same side as the eyespot is reflected onto the photoreceptors by carotenoid pigments, and when light enters the side of the cell opposite of the eyespot, the cell functions as a converging lens and directs the light onto the eyespot (Foster and Smyth 1980, Ueki et al. 2016). However, the cell needs to determine the direction from which the light entered relative to the eyespot, so carotenoid pigments block light entering from the opposite side of the cell from reaching the eyespot (Ueki et al. 2016). Previous research by Schaller and Uhl (1997) indicated that such shielding occurred due to the presence of chlorophyll and other pigments within the cell. However, recent research suggests that chlorophyll is not a sufficient shield and that carotenoid pigments adequately shield the eyespot from the lens effect of the cell (Ueki et al. 2016). This shielding ability allows the cell to determine the direction from which the light originated by allowing light to only enter from the same side as the eyespot (Ueki et al. 2016).
Once light reaches the photoreceptors, calcium ion channels in the plasma membrane surrounding the eyespot open (Foster and Smyth 1980, Harz and Hegemann 1991). The resulting changes in ion concentrations then depolarize additional calcium ion channels in the flagella which causes changes in the beating of the cis-flagellum closest to the eyespot and the trans-flagellum farthest from the eyespot (Harz and Hegemann 1991, Beck and Uhl 1994). The relative strength of each beating flagellum allows the cell to exhibit either positive phototaxis and move towards the light source, or negative phototaxis and move away from the light source (Berthold et al. 2008). Since photoreceptor stimulation is greatest when the algal cell is perpendicular to the light, the cell assumes a path that reduces the amount of light reaching the photoreceptors by moving in a direct and parallel path towards or away from the light (Foster and Smyth 1980). After the cell moves in the appropriate direction, the cell monitors the light by continually rotating to ensure that it is moving in the correct direction (Schaller et al. 1997).
The ability for eyespot-containing photosynthetic algae to interpret and respond to light stimuli is important for regulating an optimal balance of light reaching the cell (Erickson et al. 2015). Photosynthesis is an essential process for algae to survive and grow, and as a result, an excess or deficiency of light can be harmful to cellular components (Niyogi 1999, Erickson et al. 2015). Light stress in algae can result in photooxidation and the production of reactive oxygen species that can cause damage to the thylakoid photosystems as well as cellular proteins and lipids (Niyogi 1999). Therefore, the ability of eyespots to detect light and modulate flagellar movement allows algae to swim towards light when needed and away from light when the cell is exposed to a potentially harmful amount of light (Erickson et al. 2015).
C. reinhardtii contains a type A eyespot in which the eyespot is physically associated with the chloroplast but is not associated with the flagella (Dodge 1974). Other algae possess different eyespot types according to the association of the eyespot with the chloroplast and flagella. Type B eyespots are associated with both the chloroplast and flagella, and type C eyespots are not associated with the chloroplast but are associated with the flagella (Dodge 1974). Type D eyespots, present only in dinoflagellates containing diatom endosymbionts, are relict plastids of dinoflagellates’ peridinin-containing secondary chloroplasts and are associated with the flagella (Tomas and Cox 1973, Moestrup and Daugbjerg 2007, Hehenberger et al. 2014). Type E eyespots are composed of globules associated with the flagella but are not associated with the chloroplast (Lindberg et al. 2005, Siano et al. 2010). Type F eyespots are adjacent to the chloroplast and are not associated with the flagella (Craveiro et al. 2010). Type G eyespots (as described below) are associated with plastids due to the retention of plastid genomes directly within the eyespot (Gavelis et al. 2015). Note that many of these algae, especially dinoflagellates, are not model systems like C. reinhardtii, and as such their eyespot biology is not as well understood. Nevertheless, we assume that eyespot functionality in these non-model algae is similar to that in C. reinhardtii in that they respond to light as a stimulus.
GALACTOLIPIDS IN EYESPOT-CONTAINING ALGAE
The following text discusses if algae outside of the Dinophyceae contain unique galactolipids in association with the presence of an eyespot (when compared to closely related algae which do not contain eyespots). While many algal classes contain taxa with eyespots, the following discussion will focus on three classes: Chlorophyceae (type A), Cryptophyceae (varied types), and Euglenophyceae (type C) (Dodge 1974).
Chlorophyceae
Despite the large number of individual species within the Chlorophyceae, there are limited examples of eyespot-containing and eyespot-lacking genera with published galactolipid data to make an initial comparison between the two groups achievable. These include Dunaliella acidophila (Kalina) Massjuk, D. salina (Dunal) Teodoresco, D. bardawil (synonymous with D. salina), D. tertiolecta Butcher, Parietochloris incisa (H. Reisigl) Shin Watanabe, and C. reinhardtii.
Major fatty acids of Chlorophyceae, such as those found within Dunaliella, include octadecatrienoic acid [18:3(n-3)], hexadecanoic acid (16:0), octadecenoic acid [18:1(n-9)], and octadecadienoic acid [18:2(n-6)], which are also found within several other classes of algae. These fatty acids contain 3, 0, 1, and 2 double bonds, respectively. In the fatty acids 18:3(n-3), 18:1(n-9), and 18:2(n-6), the first double bond from the methyl end is in the n-3, n-9, and n-6 position, respectively. Major fatty acid biomarkers which are generally restricted to the Chlorophyceae, and a smaller number of other algal classes, include hexadecatetraenoic acid [16:4(n-3)], hexadecatrienoic acid [16:3(n-3)], and hexadecadienoic acid [16:2(n-6)] (Taipale et al. 2013).
The following have not been described as containing an eyespot. Note that where available, distributions of individual forms of MGDG (e.g., 18:3/16:4 MGDG, where the notation corresponds to the sn-1 and sn-2 fatty acids) and DGDG as relative percentages of the total galactolipids, and/or individual fatty acids as either relative percentages of either total galactolipid-associated or total fatty acids, are listed:
D. acidophila galactolipids were observed to contain high relative amounts of 18:3/16:4 MGDG (38.4%), 18:3/16:3 MGDG (10.3%), 18:3/16:2 DGDG (27.3%), 18:3/16:1 DGDG (25.0%), and 18:3/16:0 DGDG (18.7%) (Della Greca et al. 1989). Thus, the fatty acids of these galactolipids resemble those expected for this algal class.
P. incisa, an alga within the class Trebouxiophyceae but still within the same phylum as Chlorophyceae, was described as containing relatively high amounts of 16:2(n-6; 20.8%), 16:3(n-3; 11.0%), 18:2(n-6; 31.4%), and 18:3(n-3; 18.5%) fatty acids as part of MGDG, and 16:0 (34.0%), 18:2(n-6; 31.0%) fatty acids as part of DGDG, all with unknown regiochemical distributions (Bigogno et al. 2002b). In addition, Bigogno et al. (2002a) observed P. incisa to produce relatively large amounts of 16:2(n-6; 9.6%), 16:3(n-3; 23.4%), 18:2(n-6; 15.3%), and 18:3(n-3; 32.6%) fatty acids as part of MGDG and 16:0 (19.2%), 18:2(n-6; 22.2%), 18:3(n-3; 26.1%), and eicosatetraenoic acid [20:4(n-6; 12.3%)] fatty acids as part of DGDG, all with unknown regiochemical distributions. While this alga is of the class Trebouxiophyceae, the major fatty acids and fatty acid biomarkers for this class are the same as those of Chlorophyceae (Taipale et al. 2013). These fatty acids generally match those expected of other green algae.
The following do contain eyespots:
D. salina was reported as containing almost the entirety of its MGDG in the form of 18:3/16:4 (92.0%), and it also contained relatively high amounts of 18:3/16:3 (double bond positions undetermined) DGDG (46.3%), 18:3/16:0 DGDG (26.2%), and 18:3/16:4 DGDG (10.4%) (Lynch et al. 1983). Similar results were observed by Cho and Thompson (1987) in the thylakoid of D. salina as they reported relatively high amounts of 18:3/16:4 MGDG (92.5%), 18:3/16:3(n-3) DGDG (30.0%), 18:3/16:2 and 18:2/16:3(n-3) DGDG (16.7%), and 18:3/16:0 DGDG (25.6%).
The galactolipids of D. bardawil (synonymous with D. salina) were not described by Fried et al. (1982) in terms of fatty acid regiochemical (i.e., sn-1 vs. sn-2) positioning; however, they reported that MGDG was dominated by 18:3 (52.7%), 18:2 (16.2%), and 16:4 (29.7%) fatty acids. DGDG was dominated by 18:3 (25.7%), 18:2 (10.1%), and 16:0 (54.5%) fatty acids (Fried et al. 1982). These fatty acids also resemble those expected for Chlorophyceae.
D. tertiolecta was observed to possess relatively high amounts of 18:3/16:4 MGDG (63.1%) as well as 18:3/16:0 DGDG (16.6%) and 18:3/18:3 DGDG (12.3%) (Leblond et al. 2013). These fatty acids are generally characteristic of Chlorophyceae fatty acids.
The green alga C. reinhardtii was described as containing relatively large amounts of 18:3/16:4 MGDG (70%), 18:3/16:3 MGDG (16%), 18:3/18:3 DGDG (11%), 18:3/16:0 DGDG (18%), 18:2/16:0 DGDG (25%), and 18:1/16:0 DGDG (13%) (Giroud et al. 1988). As such, the fatty acids comprising chloroplast galactolipids are similar to those expected for this algal class. In addition, C. reinhardtii was observed containing relatively large amounts of MGDG containing 18:1 (15.2%), 18:2 (23.2%), and 18:3 (39.7%) fatty acids along with DGDG containing 16:0 (24.3%), 18:1 (10.6%), 18:2 (12.1%), and 18:3 (24.1%) fatty acids (Janero and Barrnett 1981); while the distributions of the MGDG and DGDG fatty acids are not noted, the fatty acids are comparable to those expected of the class Chlorophyceae.
While these species differ from the other species previously discussed since they contain eyespots, their galactolipid and fatty acid content are very similar to those of other green algae which suggests that the presence of an eyespot does not correlate with changes in galactolipid composition.
Cryptophyceae
The Cryptophyceae include multiple species with eyespots, but little galactolipid data (i.e., which galactolipids possess which fatty acids, with regiochemical specificity designated) exists for species of this class. As a result, it proves more difficult to compare galactolipids of eyespot-containing and eyespot-lacking species. However, there are total fatty acid data for Cryptophyceae algae including Cryptomonas ovata Ehrenberg, Cr. acuta Butcher, Chroomonas placoidea Butcher ex G. Novarino and I. A. N. Lucas, Ch. mesostigmata Butcher ex D. R. A. Hill, Ch. coerulea (Geitler) Skuja, Ch. caudata L. Geitler, and Hemiselmis rufescens Parke. Future research about Cryptophyceae galactolipids will clarify the distribution of fatty acids in galactolipids and which fatty acids are prominent in the chloroplast.
Major fatty acids of Cryptophyceae include 16:0, 18:3(n-3), 18:4(n-3), and eicosapentaenoic acid [20:5(n-3)] (Taipale et al. 2013, Mitani et al. 2017). Fatty acid biomarkers of Cryptophyceae include docasapentaenoic acid [22:5(n-6)] and 18:4(n-3), while noting that the 18:4 fatty acid is found in other algal classes, includes the Dinophyceae (Kumari et al. 2013, Taipale et al. 2013).
Members of the Cryptophyceae described as lacking an eyespot exist: Cr. ovata, Cr. acuta, Ch. placoidea, and Ch. caudata (Kawai and Inouye 1989). Published data include:
Cr. ovata (2 strains) was observed to contain relatively large amounts of 16:0 (16.0–16.3%), 18:3 (24.3–25.5%), 18:4 (21.3–21.4%), and 20:5 (15.0–16.4%) fatty acids (Mitani et al. 2017). These fatty acids closely resemble those expected for algae of this class. The fatty acid composition of Cr. acuta contained relatively large amounts of 16:0 (14.4%), 18:1 (13.4%), 18:3 (13.9%), and 18:4 (24.5%) fatty acids (Mitani et al. 2017).
The fatty acid composition of Ch. placoidea also contained relatively large amounts of 16:0 (24.0%), 18:1 (20.1%), 18:3 (17.3%), and 18:4 (9.8%) fatty acids (Mitani et al. 2017). These fatty acids are also typical of Cryptophyceae algae. Ch. caudata was described as containing relatively large amounts of 12:0 (12.1%), 14:0 (15.2%), 16:0 (19.6%), 18:1 (10.3%), 18:4 (13.4%), and 20:5 (9.4%) fatty acids (Mitani et al. 2017). Fatty acids of this species contain fatty acids characteristic to this class in addition to fatty acids [dodecanoic acid (12:0), tetradecanoic acid (14:0), and 18:1] not typically found in relatively large amounts.
Cryptophyceae algae described as containing eyespots include Ch. mesostigmata (Lucas 1982), Ch. coerulea (Kawai and Inouye 1989), and H. rufescens (Parke 1949). Published data include:
The fatty acid composition of Ch. mesostigmata contained relatively large amounts of 16:0 (16.1%), 18:3 (16.3%), 18:4 (20.0%), and 20:5 (13.6%) fatty acids (Mitani et al. 2017). The fatty acid composition of Ch. coerulea (three strains) also contained relatively large amounts of 16:0 (13.3–22.8%), 18:3 (11.9–14.3%), 18:4 (17.6–28.9%), and 20:5 (10.5–14.7%) fatty acids (Mitani et al. 2017). These total fatty acids are characteristic of those expected for Cryptophyceae algae.
H. rufescens was observed as containing relatively large amounts of 16:0 (21%), hexadecenoic acid 16:1(n-7; 10%), 18:4(n-3; 17%), and eicosenoic acid 20:1(n-9; 14%) fatty acids, and small amounts of 18:3(n-3; 7%) and 20:5(n-3; 8%) fatty acids (Chuecas and Riley 1969). While Ch. mesostigmata and Ch. coerulea contained total major fatty acids characteristic of Cryptophyceae, H. rufescens contained unusual fatty acids [16:1(n-7; 10%) and 20:1(n-9; 14%)] that are not considered major fatty acids for this class. However, since Ch. caudata, which lacks an eyespot, also contained unusual fatty acids (12:0, 12.1%; 14:0, 15.2%; 18:1, 10.3%), differences in total fatty acid composition and possibly galactolipid composition could be a result of factors other than the presence or absence of an eyespot.
Euglenophyceae
The class Euglenophyceae also contains algae with eyespots, and galactolipid data are minimal compared to total fatty acid data. Select Euglenophyceae algae with galactolipid and fatty acid data include Euglena gracilis G. A. Klebs, Lepocinclis acus (O. F. Müller) B. Marin and Melkonian, and Tetreutreptia pomquetensis J. L. McLachlan, M. R. Seguel and L. Fritz which all contain eyespots (McLachlan et al. 1994, Ratha et al. 2006). Major total fatty acids of this class include 16:0, 18:3(n-3), 20:5(n-3), and 22:6(n-3) (Lang et al. 2011, Taipale et al. 2013):
E. gracilis was observed to possess relatively high amounts of 18:3/16:4 MGDG (29.0%), 20:4/16:4 MGDG (14.2%), and 18:3/16:3 DGDG (10.2%) (Craig et al. 2015).
L. acus was observed to produce high relative amounts of 18:3/16:4 MGDG (31.6%), 18:3/16:2 MGDG (13.9%), and 20:4/16:4 MGDG (14.9%) (Craig et al. 2015).
T. pomquetensis was observed to possess relatively high amounts of 16:0 (13.7%), 16:4(n-3; 14.8%), 18:4(n-3; 20%), and 20:5(n-3; 14.3%) fatty acids, in the absence of galactolipid data (McLachlan et al. 1999).
The euglenoids discussed above contain eyespots. However, to our knowledge, no data exist for comparing galactolipids of eyespot-lacking euglenoids to eyespot-containing euglenoids. These eyespot-containing euglenoids contained some fatty acids typical of Euglenophyceae, but the fatty acid compositions were not as similar to each other as those observed in the previously discussed algal classes. Since the three Euglenophyceae species discussed contain eyespots, differences in fatty acid compositions may reflect other differences between the algae rather than the presence or absence of an eyespot. For example, T. pomquetensis is a cold-water alga and may contain fatty acids that aid in surviving cold water temperatures (McLachlan et al. 1999). Also, since fatty acid composition varies among eyespot-containing euglenoids, further research on euglenoids lacking eyespots can clarify whether the fatty acid compositions of euglenoids with eyespots are different than those in euglenoids without eyespots.
EYESPOT BIOLOGY IN DINOFLAGELLATES
Among eyespot-containing algae, individual phylogenetic classes (e.g., Chlorophyceae) typically possess the same eyespot type; however, eyespot variation is more distinct within the class Dinophyceae, possibly due to inherent dinoflagellate morphological and genetic diversity and the potential for dinoflagellates to acquire eyespots from symbionts (i.e., beyond the initial primary endosymbiosis) (Dodge 1984, Kreimer 1994). Among the many species of photosynthetic dinoflagellates, representative of about half of the Dinophyceae (Gaines and Elbrächter 1987), there exists only a relatively small number of taxa with eyespots despite the variation in dinoflagellate eyespot types. As reviewed by Moestrup and Daugbjerg (2007) there are (at least) five categories of eyespots (types A–E), according to protein and pigment arrangement in the eyespot as well as the eyespot’s association with the chloroplast and flagella. Per their categorization, example species according to eyespot type are (A) Peridinium willei Huitfeldt-Kaas; (B) Baldinia anauniensis Gert Hansen and Daugbjerg and Woloszynskia tenuissima (Lauterborn) R. H. Thompson; (C) members of the Tovelliaceae including Jadwigia applanata Moestrup, K. Lindberg and Daugbjerg, Tovellia coronata (Woloszynska) Moestrup, K. Lindberg and Daugbjerg, and Tovellia sanguinea Moestrup, Gert Hansen, Daugbjerg, G. Flaim and d’Andrea; (D) dinoflagellates with a diatom endosymbiont (“dinotoms”), including species within the genus Durinskia and Kryptoperidinium foliaceum (F. Stein) Lindemann; and (E) members of the Suessiales including Polarella glacialis M. Montresor, G. Procaccini and D. K. Stoecker, Protodinium simplex Lohmann (formerly known as Gymnodinium simplex), and Woloszynskia halophila (Biecheler) M. Elbrächter and A. Kremp, which is also known as Biecheleria halophila (Biecheler) Moestrup, Lindberg and Daugbjerg.
Additional members of these groups not mentioned by Moestrup and Daugbjerg (2007) include: (A) Amphidinium cupulatisquama M. Tamura and T. Horiguchi (Tamura et al. 2009), Gertia stigmatica K. Takahashi, Benico, Wai Mun Lum and Iwataki (Takahashi et al. 2019), Margalefidinium polykrikoides (Margalef) F. Gómez, Richlen and D. M. Anderson (Iwataki et al. 2010) and Margalefidinium fulvescens (M. Iwataki, H. Kawami and Matsuoka) F. Gómez, Richlen and D. M. Anderson (Iwataki et al. 2007); (B) members of the genus Borghiella (Moestrup et al. 2009) and Dactylodinium pterobelotum K. Takahashi, Moestrup and Iwataki (Takahashi et al. 2017); and (E) Symbiodinium microadriaticum LaJeunesse (LaJeunesse 2017).
These five categories have been expanded upon by Gavelis et al. (2015), who described the ultrastructure of yet more complex eyespots known as ocelloids within Nematodinium sp. and Erythropsidinium agile (Hertwig) P. C. Silva. Such eyespots contain structures resembling a lens, cornea, and retina (Greuet 1967) and are also present in Proterythropsis, Greuetodinium, and Warnowia species, with all ocelloid-containing dinoflagellates classified within the family Warnowiaceae (Hoppenrath et al. 2009 and references therein). For the purposes of this paper, these are labeled as type G. Of these seven categories, all of the members, except the dinotoms (Gagat et al. 2014), have been demonstrated to have or are assumed to have peridinin-containing, secondary plastids of red algal origin (Keeling 2010, Dorrell and Howe 2015, Waller and Kořený 2017). For a visual summary of eyespot architecture within dinoflagellates, the reader is recommended to consult the corresponding figure within Gavelis et al. (2015).
There are additional eyespot-containing dinoflagellates whose galactolipid composition has not previously been analyzed, but future research aiming to do so will further contribute to the understanding of the relationship between galactolipids and eyespots. These species as according to eyespot type include: type A, Caladoa arcachonensis Z. Luo, K. N. Mertens & H. F. Gu (Luo et al. 2019), Dactylodinium arachnoides W. M. Lum, K. Takahashi, Takayama & Iwataki (Lum et al. 2019), Chimonodinium lomnickii (Woloszynska) Craveiro, Calado, Daugbjerg, Gert Hansen & Moestrup (Craveiro et al. 2011), Palatinus apiculatus (Ehrenberg) Craveiro, Calado, Daugbjerg & Moestrup (Craveiro et al. 2009), Naiadinium polonicum (Woloszynska) Carty (Craveiro et al. 2015), and Paragymnodinium stigmaticum Yokouchi, Onuma & Horiguchi (Yokouchi et al. 2018); type E, Ansanella granifera H. J. Jeong, S. H. Jang, Moestrup & N. S. Kang (Jeong et al. 2014), Ansanella natalensis (T. Horiguchi & R. N. Pienaar) Dawut, Sym & T. Horiguchi (Dawut et al. 2018), Asulcocephalium miricentonis Kazuya Takahashi, Moestrup & M. Iwataki (Takahashi et al. 2015), Biecheleria baltica Moestrup, Lindberg & Daugbjerg (Moestrup et al. 2009), Biecheleria brevisulcata K. Takahashi & Iwataki (Takahashi et al. 2014), Biecheleria pseudopalustris (J. Schiller) Moestrup, K. Lindberg & Daugbjerg (Moestrup et al. 2009), Biecheleria tirezensis S. Fraga, N. Raho, J. P. Abad & I. Marín (Raho et al. 2018), Biecheleriopsis adriatica Moestrup, Lindberg & Daugbjerg (Moestrup et al. 2009), Leiocephalium pseudosanguineum Kazuya Takahashi, Moestrup & M. Iwataki (Takahashi et al. 2015), and Yihiella yeosuensis S. H. Jang, H. J. Jeong, Moestrup & N. S. Kang (Jang et al. 2017); type F, Sphaerodinium cracoviense Woloszynska (Craveiro et al. 2010) and Sphaerodinium polonicum var. tatricum Woszolynska (Pandeirada et al. 2021).
Each of the seven categories of dinoflagellate eyespots has some combination of carotenoids and/or protein lenses, with varying levels of complexity. As ocelloid eyespots resembling animal eyes, type G is the most complicated in terms of architecture. Of these seven categories of dinoflagellate eyespots, five (A, B, D, F, and G) are considered per Moestrup and Daugbjerg (2007), Craveiro et al. (2010), and Gavelis et al. (2015) to have a direct association with plastid membrane(s). However, according to Hehenberger et al. (2014) and Pienaar et al. (2007), dinotoms contain an eyespot that is not directly associated with the diatom-derived plastid. Nonetheless, dinotoms contain peridinin-containing plastid-derived genes, and the eyespot could be a relict peridinin-containing secondary plastid (Hehenberger et al. 2014), although the evidence is equivocal. Likewise, while E. agile contains a type G eyespot and lacks a chloroplast, it has been found to contain genes related to photosynthesis which suggest that the eyespot could be a relict plastid (Gavelis et al. 2015). According to Moestrup and Daugbjerg (2007), types A, B, and D contain one or more layers of lipid droplets, presumably carotenoids, within a plastid structure, while according to Gavelis et al. (2015) type G does as well. Type F contains an oil layer adjacent to plastid membranes (Craveiro et al. 2010). In addition, according to Moestrup and Daugbjerg (2007), type B is similar in structure to type A, but it contains layer(s) of crystalline protein adjacent to the eyespot; types F and G do as well (Craveiro et al. 2010, Gavelis et al. 2015).
Type C and E eyespots appear to lack association with plastid membranes. According to Moestrup and Daugbjerg (2007), type C is composed of a layer of lipid droplets not contained within a plastid membrane, and type E is a set of stacked protein layers not found adjacent to a plastid membrane. In addition to structural variations, eyespots also differ in their placement within the cell relative to the flagella. As described by Dodge (1974), type A eyespots are not adjacent to the flagella, but both types B and C are adjacent to the flagella. As observed in multiple dinotoms, type D eyespots are adjacent to the flagella (Tomas and Cox 1973). Type E eyespots are adjacent to the flagella since the eyespots are located in the sulcus region of the cell from which the flagella emerge (Kremp et al. 2005, Moestrup et al. 2009, Siano et al. 2010).
GALACTOLIPIDS IN EYESPOT-CONTAINING DINOFLAGELLATES
Over the past decade, extensive characterization has been made of MGDG and DGDG of both peridinin-containing dinoflagellates with secondary plastids of red algal origin and of dinoflagellates with aberrant plastids. These two galactolipids are important structural lipids in plastid membranes (Murata and Siegenthaler 1998), and are thus conserved features of virtually every photosynthetic organism. The diversity of MGDG and DGDG forms (as based on the associated fatty acids) in both peridinin-containing dinoflagellates with secondary plastids of red algal origin, and dinoflagellates with aberrant plastids, has recently been reviewed by Leblond et al. (2019). Briefly, the peridinin-containing dinoflagellates have been found to segregate into two clusters according to Gray et al. (2009b). Cluster 1 represents dinoflagellate taxa with C18/C18 (sn-1/sn-2) fatty acid regiochemistry, while Cluster 2 represents those with C20/C18 regiochemistry. The C18 fatty acids are octadecapentaenoic [18:5(n-3)] and octadecatetraenoic [18:4(n-3)] acid, while the C20 fatty acid is 20:5(n-3).
Of the secondary plastid, peridinin-containing dinoflagellates with eyespots listed above, the following have had their galactolipids characterized: Per. willei (type A), Borghiella dodgei Moestrup, Gert Hansen and Daugbjerg and Borghiella tenuissima (Lauterborn) Moestrup, Gert Hansen and Daugbjerg (type B), J. applanata and T. coronata (type C), and Pro. simplex, S. microadriaticum, and W. halophila (type E). The aberrant plastid-containing dinoflagellate K. foliaceum (type D) has also had its galactolipids characterized. To date P. willei is the only type A dinoflagellate with a published galactolipid composition (Gray et al. 2009b) and K. foliaceum is the only type D dinoflagellate with published galactolipid data (Leblond and Lasiter 2009). Our research has contributed to the galactolipid data of eyespot-containing dinoflagellates by identifying the galactolipids present in M. polykrikoides (type A) and Durinskia baltica (Levander) Carty and Elenor R. Cox (type D).
The eyespots described below that are associated with the chloroplast include type A (M. polykrikoides and Per. willei) and type B (B. dodgei and B. tenuissima). Type A eyespots tend to be situated close to the edge of the chloroplast, and type B eyespots tend to be situated at the front of the chloroplast (Dodge 1974). Eyespots described above that are not associated with the chloroplast include type C (J. applanata and T. coronata) and type E (Pro. simplex, S. microadriaticum, and W. halophila). Type D (K. foliaceum and D. baltica) eyespots have been described by Moestrup and Daugbjerg (2007) to be closely associated with the chloroplast but have also been described by Pienaar et al. (2007) and Hehenberger et al. (2014) to not be closely associated with the diatom-derived chloroplast. A summary of the galactolipid compositions of algae possessing eyespots are as follows.
Type A
We have examined two isolates of M. polykrikoides (ARC 47 and ARC 169 from the Algal Resources Collection, Wilmington, NC, USA) according to the growth conditions and galactolipid characterization methodologies put forth in our previous papers on MGDG and DGDG in dinoflagellates, and these isolates were observed to possess 20:5/18:5 MGDG (m/z 817, approximately 45%) and 20:5/18:5 DGDG (m/z 979, approximately 55%). Both strains also possessed trace amounts of 18:5/18:5 MGDG (m/z 789) and 18:2/16:0 DGDG (m/z 939) yet showed no discernable difference in galactolipid composition from other dinoflagellates in the C20/C18 galactolipid cluster described earlier.
Per. willei was found by Gray et al. (2009b) to reside in the C20/C18 cluster with 20:5/18:5 MGDG, 20:5/18:4 MGDG, and 20:5/18:4 DGDG making up its galactolipid complement. The galactolipid composition of Per. willei as determined by Anesi et al. (2016) was marked by 38:10 MGDG, 38:9 MGDG, and 38:9 DGDG. Assuming that these are the 20:5/18:5 MGDG, 20:5/18:4 MGDG, and 20:5/18:4 DGDG, respectively, listed in their supplementary data, to match the results of Gray et al. (2009b), this would also place this particular isolate of Per. willei firmly within the C20/C18 cluster.
Type B
B. dodgei and B. tenuissima were both found by Anesi et al. (2016) to possess 36:9 MGDG and 36:9 DGDG as the predominant galactolipids. Using the regiochemical data presented in the supplementary data for this study to identify these galactolipids as 18:5/18:4 MGDG and 18:5/18:4 DGDG, respectively, this places these species firmly within the C18/C18 cluster.
Type C
J. applanata was observed by Anesi et al. (2016) to possess high relative amounts of 38:10 MGDG, 38:9 MGDG, and 38:9 DGDG. These represent 20:5/18:5 MGDG, 20:5/18:4 MGDG, and 20:5/18:4 DGDG, respectively, thus placing J. applanata firmly in the C20/C18 cluster.
T. coronata was observed by Anesi et al. (2016) to possess high relative amounts of 36:9 MGDG and 36:9 DGDG, representing 18:5/18:4 MGDG and 18:5/18:4 DGDG, respectively. This places T. coronata firmly within the C18/C18 cluster.
Type D
The previous comparisons of galactolipid data are made between peridinin-containing dinoflagellates with eyespots. However, dinotoms contain a diatom-derived aberrant plastid that arose via tertiary endosymbiosis, so this group does not fit into the galactolipid clusters for peridinin-containing plastids (Chesnick et al. 1997). To date, there are a handful of genera known to reside within the dinotom group. These reside monophyletically within the Peridiniales and include species within Blixaea, Durinskia, Galeidinium, Kryptoperidinium, Peridiniopsis, Peridinium, and Unruhdinium (Yamada et al. 2017, Kretschmann et al. 2018), noting that not every species of Peridinium is a dinotom (Guiry and Guiry 2020). While Blixaea, Durinskia, Galeidinium, Kryptoperidinium, and Unruhdinium differ moderately with respect to morphological features (Kretschmann et al. 2018), the most striking distinction between these genera is that the origin of the dinotom chloroplast is not monophyletic but polyphyletic (Yamada et al. 2017). Specifically, depending on the dinotom the chloroplast is derived from either the centric diatom genera Chaetoceros, Cyclotella, or Discostella, or the pennate diatom genus Nitzschia (Yamada et al. 2017), indicating that acquisition of the diatom tertiary plastid was not a single event. K. foliaceum, a single representative of the type D eyespot of dinotoms, to our knowledge, was the only dinotom available from culture collections for study of its galactolipids, at the time of its examination. The lipid biochemistry properties of dinotoms ideally should be established on data from more than one organism.
Unfortunately, to our knowledge at the current time, only one other dinotom, the marine species D. baltica is commercially available for continued study of dinotom galactolipids. Both D. baltica and K. foliaceum are Nitzschia-type marine dinotoms with tertiary plastids extracted from the diatom genus Nitzschia, and the two are placed within a group of peridinioid dinoflagellates as based on a gene for small subunit ribosomal RNA (SSU rRNA) (Yamada et al. 2017). However, the work of Imanian et al. (2010) indicates that outside contributors (e.g., genetic material from algae other than diatoms) to the current structures of their respective plastid, and possibly nuclear, genomes may appear to have been divergent over the course of evolutionary time. Additionally, the data of Yamada et al. (2017) indicate that, although both D. baltica and K. foliaceum are Nitzschia-type marine dinotoms, they likely have different species of Nitzschia as their tertiary endosymbionts. Thus, it remains an open question whether the galactolipid compositions of these two dinotoms may be divergent as well. Recognizing that galactolipids are the end products of many enzymatic steps (Makshakova et al. 2020), the galactolipid compositions of aberrant plastid dinoflagellates have been shown to be a combination of traits imparted by common peridinin-containing dinoflagellates and the secondary or tertiary endosymbiont (Leblond and Lasiter 2009, Leblond et al. 2019). The galactolipid compositions of the dinotoms are:
K. foliaceum has been found to have forms of MGDG and DGDG with 20:5(n-3) in the sn-1 position and the multiply-unsaturated C16 fatty acids 16:3 and 16:2 (double bond positions undetermined) in the sn-2 position, in a manner identical to the pennate diatom, Navicula perminuta Grunow (Leblond and Lasiter 2009).
We have examined one isolate of D. baltica (ARC 210), and have observed it to possess 20:5/16:4 MGDG (m/z 791; relative percent abundance approximately 25%), 20:5/16:3 MGDG (m/z 793; relative percent abundance approximately 65%), and 20:5/16:2 DGDG (m/z 957; relative percent abundance approximately 10%).
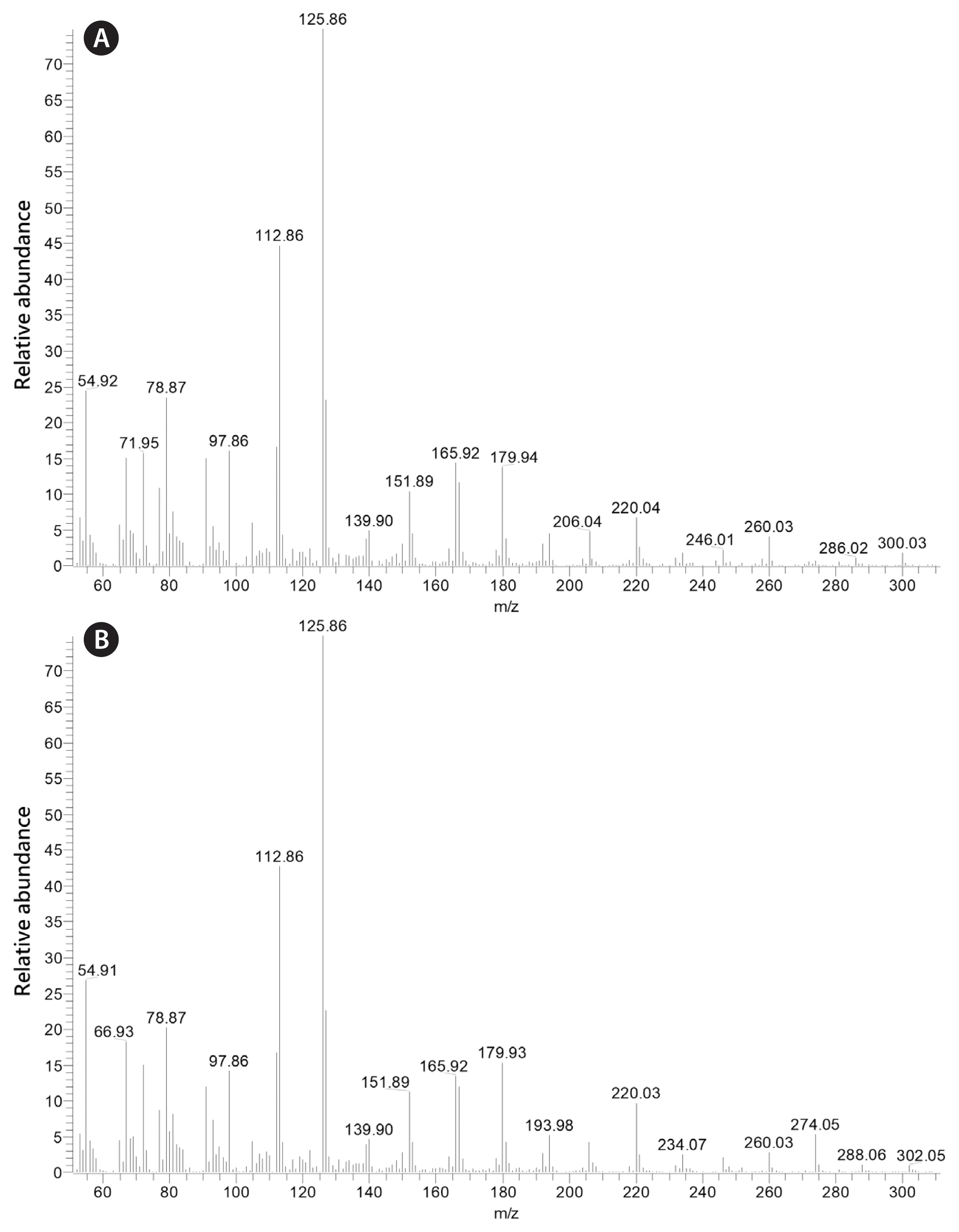
It is important to note that while the fatty acid composition of K. foliaceum and D. baltica are very similar, MGDG containing the 16:4 fatty acid identified in D. baltica was not identified by Leblond and Lasiter (2009) in K. foliaceum. The mass spectra of this galactolipid and 20:5/16:3 MGDG are shown in Fig. 1. Through this research, the dinotom D. baltica has been found to contain MGDG with 20:5 in the sn-1 position with 16:3 and 16:4 in the sn-2 position. D. baltica has also been found to contain DGDG with 20:5 in the sn-1 position and 16:2 in the sn-2 position. The placement of the first double bond of the 16:4 fatty acid was not determined, but 16:4(n-3) is common among green algae, and 16:4(n-1) has been identified in some diatoms (Dunstan et al. 1993, Taipale et al. 2013, Johansson et al. 2019). In addition, 20:5/16:4 MGDG was identified in the pennate diatom Phaeodactylum tricornutum Bohlin (Dodson et al. 2013). The mass spectra of 4,4-dimethyloxazoline (DMOX) derivatives of 16:4(n-1) and 16:3(n-4) fatty acids are shown in Fig. 2 (see Leblond et al. 2019 and references therein for the use of DMOX derivatives to determine fatty acid double bond positions).
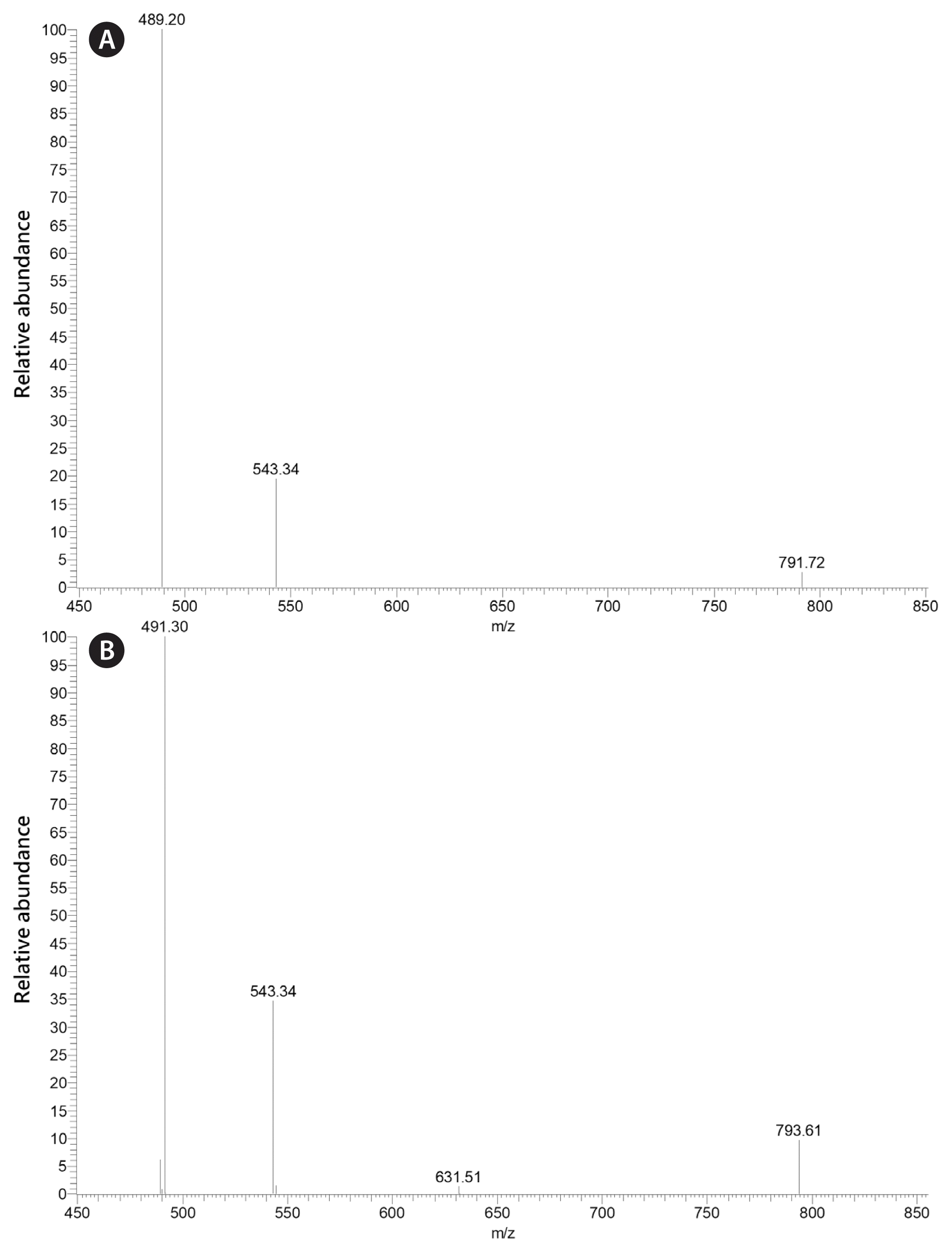
Positive-ion electrospray ionization/mass spectrometry/mass spectrometry spectra of 20:5/16:4 MGDG (m/z 791) (A) and 20:5/16:3 (m/z 793) (B) from Durinskia baltica ARC 210. In (A), the m/z 489 ion represents loss of the 20:5 fatty acid from the sn-1 position, while the m/z 543 ion represents loss of the 16:4 fatty acid from the sn-2 position. In (B), the m/z 491 ion represents loss of the 20:5 fatty acid from the sn-1 position, while the m/z 543 ion represents loss of the 16:3 fatty acid from the sn-2 position.
Type E
P. simplex, Protodinium sp., and three isolates S. microadriaticum, along with one isolate of Symbiodinium sp., were found by Gray et al. (2009b) to reside in the C18/C18 cluster. All isolates had 18:5/18:5 MGDG, 18:5/18:4 MGDG, 18:5/18:4 DGDG, and 18:4/18:4 DGDG as their galactolipid complement.
A single isolate of W. halophila was examined by Gray et al. (2009a) and found to possess 18:5/18:5 MGDG, 18:5/18:4 MGDG, 18:5/18:5 DGDG, and 18:5/18:4 DGDG, along with a small relative percentage of 18:1/14:0 DGDG. Note that this isolate is cold-adapted and was grown at a lower temperature than the isolates examined in the Gray et al. (2009b) study, and that the increased level of unsaturation in 18:5/18:5 DGDG compared to the other type E eyespot dinoflagellates (which were not observed to possess this particular galactolipid) is likely a temperature-induced modulation.
In order to provide a graphical representation of the chemotaxonomic relationships (as based on MGDG and DGDG compositions) of the eyespot- and peridinin-containing dinoflagellates (types A–C and E) described above to non-eyespot-containing, peridinin-containing dinoflagellates, all with secondary plastids, a clustergram (Fig. 3) was created using the Primer-e software package (Quest Research Limited, Auckland, New Zealand). This clustergram is based on a Bray-Curtis similarity resemblance matrix of untransformed relative percentage data from the published works of Gray et al. (2009a, 2009b) and Anesi et al. (2016), and the data for M. polykrikoides ARC 47 and ARC 169 described above. Note that D. baltica ARC 210 was omitted from this analysis because it possesses an aberrant plastid and as such has very different MGDG and DGDG compositions than peridinin-containing dinoflagellates with secondary plastids. The following points are evident in this clustergram and reiterate observations presented above:
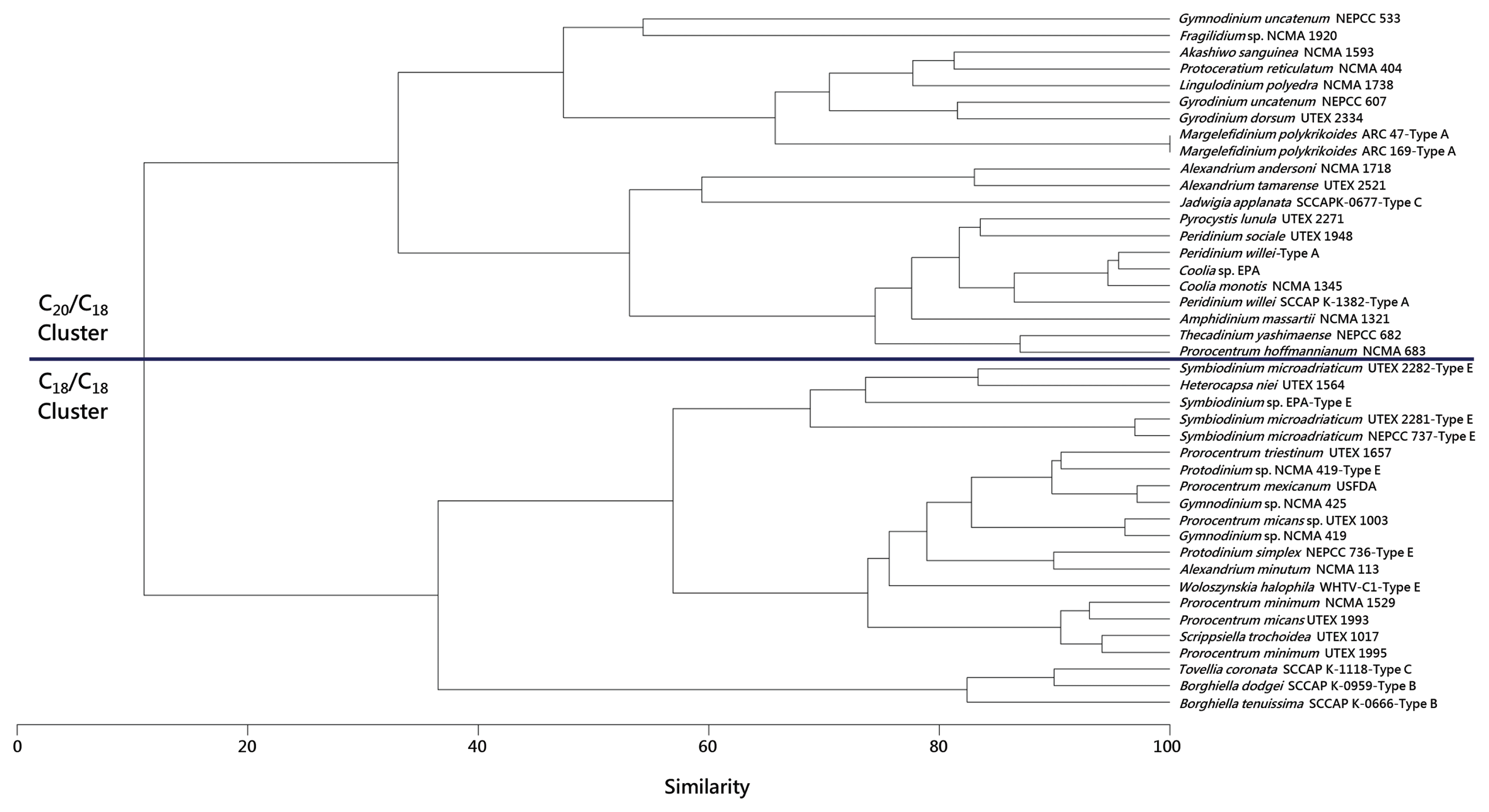
Bray-Curtis similarity clustergram of eyespot- and non-eyespot-containing dinoflagellates as based on galactolipid compositions. Most of the dinoflagellates listed are those examined by Gray et al. (2009b); while the culture collection numbers are the same as that study, some of the organism names are now different per the culture collections’ lists of species. The data for Woloszynskia halophila WHTV-C1 have emanated from Gray et al. (2009a), and the data for Borghiella dodgei SCCAP-K0959, Borghiella tenuissima SCCAP K-0666, Jadwigia applanata SCCAP K-0677, and Tovellia coronata SCCAP K-1118 emanate from the publicly available supplementary data of Anesi et al. (2016). The data for Margalefidinium polykrikoides ARC 47 and ARC 169 are presented in this work. All eyespot-containing taxa are labeled. The scale at the bottom of the clustergram represents Bray-Curtis similarity.
The dinoflagellates listed are divided into the same C18/C18 and C20/C18 clusters originally observed by Gray et al. (2009b).
All type A eyespot taxa were located within the C20/C18 cluster, and all type B and type E taxa were located in the C18/C18 cluster.
Type C J. applanata was located in the C20/C18 cluster, while type C T. coronata was located in the C18/C18 cluster.
Importantly, whether found in the C18/C18 or the C20/C18 cluster, eyespot-containing dinoflagellates were within the same levels of similarity as non-eyespot-containing taxa. For example, in the C20/C18 cluster the lowest level of similarity was approximately 35% amongst non-eyespot-containing taxa (i.e., from the top to the bottom of the cluster); all eyespot-containing taxa were within this level of similarity. The same type of phenomenon also occurred within the C18/C18 cluster.
In order to express the driving factors behind the clustering patterns, a shade plot (heat map, Fig. 4) was also generated using Primer-e with the clustergram of Fig. 3 positioned along the Y-axis and the relative percentage data of Gray et al. (2009a, 2009b) and Anesi et al. (2016) displayed using the color scale. As displayed in this figure, all eyespot- and non-eyespot-containing dinoflagellates within a particular cluster shared the same general set of major forms of MGDG and DGDG. In the C18/C18 cluster, these forms were 18:5/18:5 MGDG and DGDG, 18:5/18:4 MGDG, and 18:4/18:4 DGDG. In the C20/C18 cluster these forms were 20:5/18:5 MGDG and DGDG, and 20:5/18:4 MGDG and DGDG.
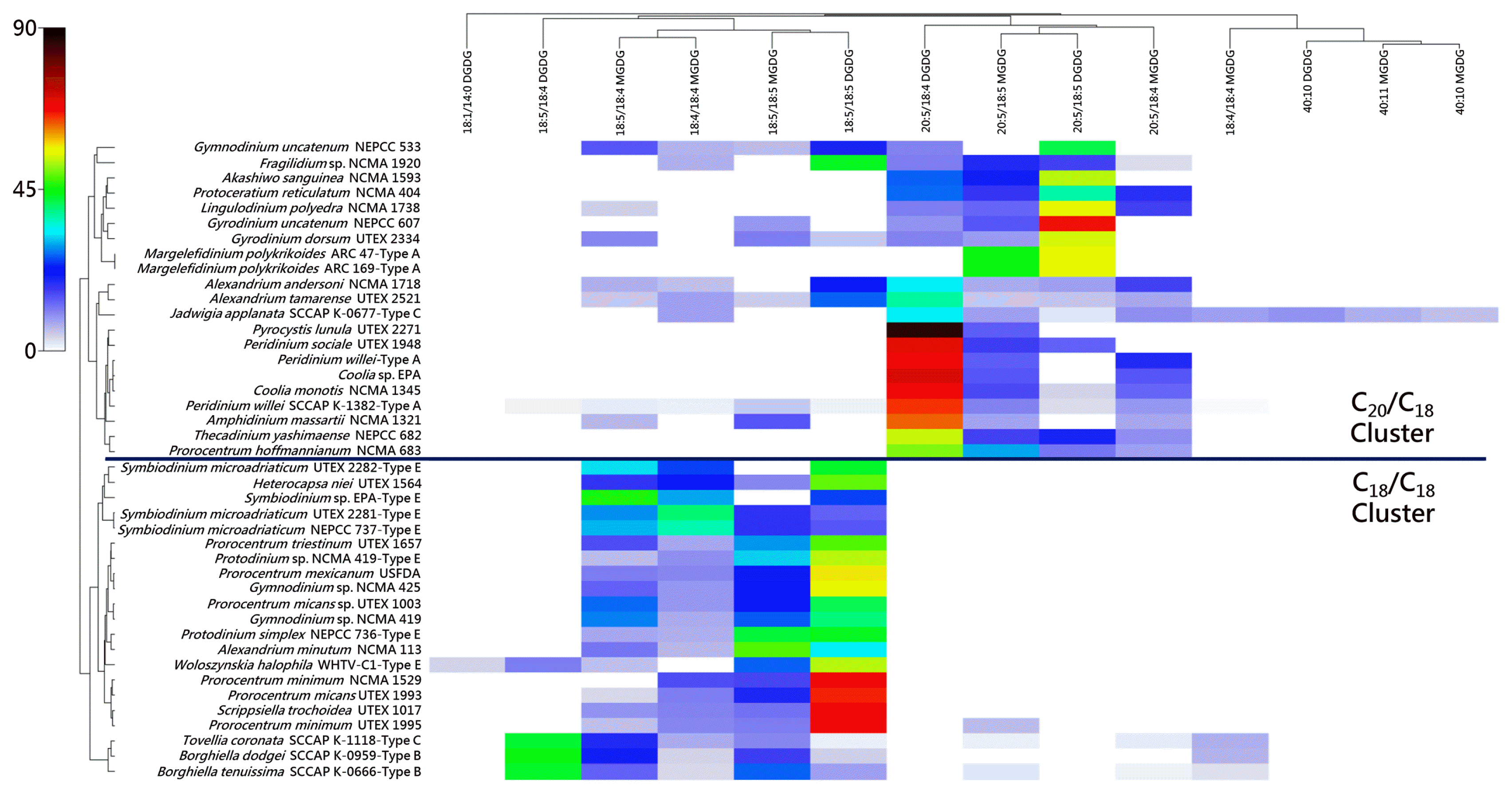
Bray-Curtis similarity clustergram of eyespot- and non-eyespot-containing dinoflagellates couple with shade plot of monogalactosyldia-cylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) relative percentages. The forms of MGDG and DGDG found in these dinoflagellates are represented along the X-axis, and the relative percentage values of each galactolipid correspond to the color scale shown to the upper left. All eyespot-containing taxa are labeled.
It should be mentioned as a point of clarification that 20:5/18:5 and 20:5/18:4 DGDG, for example, in actuality represent two versions of the same lipid, with the level of total unsaturation (but not the total number of fatty acid carbons) being modulated according to culture conditions such as temperature (see Leblond et al. 2013 for further description). Therefore, although in both clusters there were other minor forms of MGDG and DGDG such as what are represented for J. applanata and W. halophila, the major galactolipid forms were shared across the eyespot- and non-eyespot-containing dinoflagellates in a given cluster.
CONCLUSION
Eyespot-containing algae within algal classes other than the Dinophyceae do not appear to contain distinct galactolipids compared to eyespot-lacking dinoflagellates; however, future galactolipid research of classes including the Cryptophyceae and Euglenophyceae will clarify the positional distribution of fatty acids in chloroplast galactolipids. Because every one of the secondary plastid, peridinin-containing dinoflagellates with eyespots was indistinguishable from other eyespot-lacking members of the respective C18/C18 or C20/C18 clusters, with galactolipids virtually identical to several dinoflagellates not known to possess eyespots, we conclude that the presence of an eyespot is not related to any appreciable alteration of galactolipid composition. In addition, the galactolipids of the aberrant plastid-containing dinotoms K. foliaceum and D. baltica (with type D eyespots) produce very similar galactolipids despite possibly having different species of Nitzschia as their tertiary endosymbionts (Yamada et al. 2017).
We initially hypothesized that the presence of eyespots would be correlated with alterations of the overall composition of these galactolipids away from what has been observed in eyespot-lacking taxa in order to facilitate placement of their protein and/or lipid components within and/or adjacent to a chloroplast membrane(s). The similar galactolipids of peridinin-containing dinoflagellates with and without eyespots are despite eyespot types A, B, F, and G residing within or being associated with the chloroplast membrane (Dodge 1974, Craveiro et al. 2010, Gavelis et al. 2015). Thus, this is an indication that evolution of the different types of dinoflagellate eyespots, assuming that they came about post-development of the dinoflagellate secondary plastid, is not related to changes in galactolipid composition despite the close association of eyespots with plastid membranes.
Abbreviations
DGDG
digalactosyldiacylglycerol
DMOX
4,4-dimethyloxazoline
MGDG
monogalactosyldiacylglycerol
Notes
The authors declare that they have no potential conflicts of interest.