INTRODUCTION
Changes in the relative fraction of inorganic carbonate species in the ocean driven by ocean acidification (OA) account for many ecological consequences that result from the biochemical and physiological responses of marine organisms to climatic change (Doney et al. 2009, Sunday et al. 2017). There have been many attempts to extrapolate the ecological effects of acidified seawater in small and tightly controlled laboratory experiments to great biological diversity and structural complexity of natural ecosystems. The superlative environmental conditions of natural carbon dioxide (CO2) and hydrogen ion activity (pH) gradients (e.g., volcanic CO2 vents) are truly appropriate to study the effects of naturally acidified seawater on marine communities (Hall-Spencer et al. 2008, Kroeker et al. 2011). Volcanic vents influence carbonate chemistry, creating a gradient of decreasing pH along a shallow rocky bottom and allow the integration of species interactions and seasonal cycles, unlikely to be achieved under laboratory settings (Ravaglioli et al. 2017). With few exceptions, on-site mesocosm studies have been performed persistently to assess the response of marine organisms to changes in carbonate chemistry (Petersen et al. 2009, Barry et al. 2010, Stewart et al. 2013).
The experimental manipulation of an ecosystem in a mesocosm can be used to generate a variety of realistic responses to OA conditions; however, it is difficult to manipulate the experimental conditions to the extent demanded by the dynamic ranges in natural environments (Duarte et al. 2015). Small-scale experiments to predict ecosystem responses, including laboratory mesocosms, are easy to control, but are not realistic (Petersen et al. 2009, Jeong et al. 2016). Meanwhile, laboratories or field mesocosm facilities have been recognized as powerful platforms for evaluating the ecological responses of marine organisms to a wide variety of natural and anthropogenic environments because they contain various ecosystem constituents, including biological and chemical processes (Riebesell et al. 2008).
There are several in situ mesocosm studies on the effect of simulated future conditions on the pelagic primary production and food web associated with biogeochemical cycles (Kim et al. 2008, Riebesell et al. 2013). Conversely, predicting the impact of carbonate chemistry changes in a high CO2 world on benthic communities is difficult because of the intricate interactions between biotic and abiotic components (Widdicombe et al. 2010). Numerous OA studies on benthic organisms rely mostly on laboratory experiments at small spatiotemporal scales (Widdicombe et al. 2010). Few studies have been conducted using an outdoor benthic mesocosm (Burrell et al. 2015, Wahl et al. 2015); however, the facilities are considered to be complicated to manipulate CO2 concentration and are expensive to install and maintain. In addition, several studies of benthos have used an enclosed tank system without seawater flow that utilized certified mixture of air-CO2 gases to create elevated CO2 conditions (Jokiel et al. 2008, Jiang et al. 2010, Kang and Kim 2016).
It is difficult to maintain consistent CO2 measurements in an open continuous flow-through system containing running seawater. This may not be able to afford the necessary insight regarding the patterns and processes of benthos in relation to experimental manipulations due to several limitations such as nutrient and trace element depletion, decomposition of organic matter, and so on. We expect that an on-site benthic mesocosm will provide many benefits for estimating benthic-pelagic coupling for biogeochemical exchanges and biological interactions. In doing so, it will allow for the easy manipulation of desired CO2 concentrations by using the seawater dilution ratio between ambient and CO2-saturated seawaters, whenever it is required.
MATERIALS AND METHODS
Mesocosm facility and pCO2 perturbation system
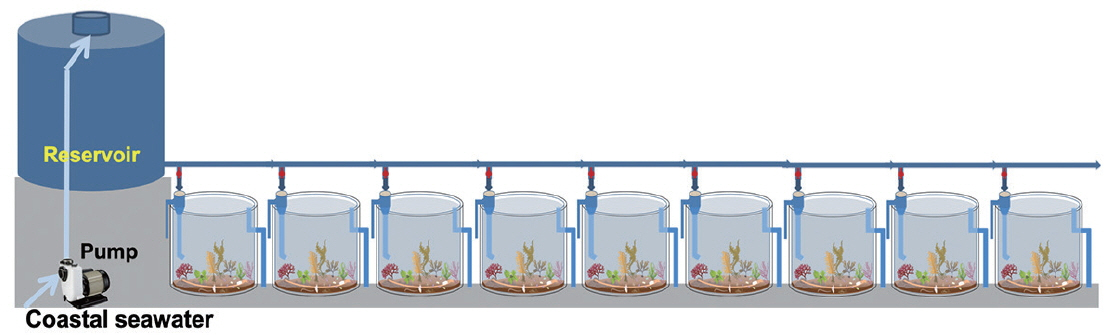
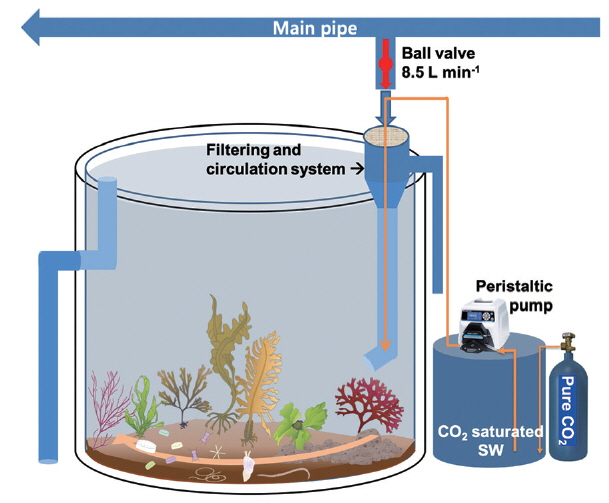
The mesocosm was installed near the rocky shore of Namhae-gun, South Sea of Korea (34°48′30″ N, 127°49′37″ E), and consisted of cylindrical acrylic tanks (1.2 m in diameter and 1.2 m in height) that were transparent and lidless (Figs 1 & 2). Coastal seawater was sequentially pumped at the rate of 100 L min−1 into the reservoir (5 t capacity) through a 300 μm mesh screen to remove large-sized organisms, detritus, and sediments. The pumps were designed to be self-priming (PA-1688, Agricultural & Industrial Pumps, 2 Horsepower Capacity; Hanil Electric Co., Daejeon, Korea) and able to pump seawater used earlier for CO2 manipulation to the reservoir. The inflow rate of seawater from the reservoir to the mesocosm tanks remained constant during the experimental period. It was controlled by seawater inlet pipes with a pit diameter (approximately 9 L min−1 with 7 mm pit diameter). After the tank was filled with a 10 cm layer of sand and gravel taken from a nearby site, seawater was added through the water supply pipe until the outlet pipe overflowed (Fig. 2). The volume of seawater in each tank was approximately 1,250 L. Nine tanks were arranged in a row in a north-south direction to achieve same lighting conditions during the daytime. To attain and maintain the target pCO2 or pH values in tank seawater, CO2-saturated seawater was added to the seawater through the inflow pipe using a high-precision peristaltic pump (Masterflex Pump 7523–57; Cole-Parmer Instrument Co., Niles, IL, USA). CO2-saturated seawater was generated by bubbling commercially prepared pure CO2 gas, which achieved the seawater pH to drop below 5.
Regulation of carbonate chemistry by dilution ratio
Varied pH or pCO2 levels were manipulated by dilution ratios of CO2-saturated seawater to ambient seawater. The flow rates of CO2-saturated seawater flowing through the inlet pipe were precisely controlled by peristaltic pumps at nine steps with a range of 0–85 mL min−1, where ambient seawater flowed into the mesocosm tanks constantly at a rate of 8.5 L min−1. Seawater samples were collected and analyzed for carbonate chemistry from each tank every 30 min (9 times) over a 4-h period, after dilution was initiated. This experiment was designed to determine the optimal time for constructing stable pH or pCO2 levels, and was conducted in September 2013 when seawater temperatures ranged from 26 to 27°C with a salinity of 31 psu.
Daily fluctuation of carbonate chemistry by dilution ratio
Ambient and two elevated pCO2 levels were created to monitor daily fluctuations of carbonate chemistry in coastal seawater. These conditions were constructed by two inflow rates of CO2-saturated seawater, specifically 25 mL min−1 (dilution ratio = 0.003 and 0.2 pH unit dropped) and 50 mL min−1 (dilution ratio = 0.006 and 0.5 pH unit dropped). The flow rate of ambient seawater was fixed at approximately 9 L min−1. Using the siphon tubing, seawater samples were carefully collected from the mesocosm tanks at a depth of 0.5 m every 3 h over a 24-h period. This experiment was performed with triplicate mesocosm tanks for each CO2 condition (n = 3) and was conducted on Oct 24–25, 2013, when seawater temperatures ranged from 18 to 21°C with a salinity of 29 psu.
Determination of carbonate chemistry
Carbonate chemistry was analyzed based on the standard operation procedure for ocean CO2 measurements (Dickson et al. 2007). Seawater samples were collected by siphoning from the mid-depths of the tank and filled 500 mL borosilicate airtight glass bottles (1,500–500 Pyrex; Corning, NY, USA). The bottles were immediately closed with high quality vacuum grease (Apiezon M grease; M&I Materials Ltd., Manchester, UK) after poisoning them with mercuric chloride (HgCl2), and keeping them in a cold room prior to analysis.
The total alkalinity (AT) and pH of seawater samples were analyzed within two days after acquisition and were used to calculate seawater carbonate chemistry with CO2SYS software (Lewis and Wallace 1998). The AT was measured in the laboratory by a potentiometric acid titration system at a constant temperature of 25°C. This system consists of a Metrohm 765 Dosimat titrator (Metrohm, Zofingen, Switzerland) and Orion 920A pH meter connected with a ROSS half-cell pH electrode (8101BNWP) and a reference electrode (Orion 900200) (Thermo Fisher Scientific, Waltham, MA, USA). Potentiometry measurements of electromotive force of the pH electrode and the volume of added hydrochloric acid (HCl) were acquired semi-automatically using a Q-basic program (Millero et al. 1993).
Seawater pH was determined with spectrophotometric measurements using a high-resolution spectrophotometer (Agilent 8453 UV-Visible Spectrophotometer; Agilent Technologies, Pal Alto, CA, USA). Using a siphon tube, the samples were filled into a 10 cm path length cuvette without air bubbles, and then, absorbance was measured with or without adding an indicator dye at wavelengths of 434, 578, and 730 nm and a constant temperature of 25°C. The perturbation of the indicator metacresol purple (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) on the pH was used (Dickson 1993). The precision of pH and AT was checked using certified reference materials (certified by Dickson, A., Scripps Institution of Oceanography, San Diego, CA, USA) and was approximately ±0.004 unit for pH and ±2 μmol kg−1 for AT.
RESULTS
Regulation of carbonate chemistry by dilution ratio
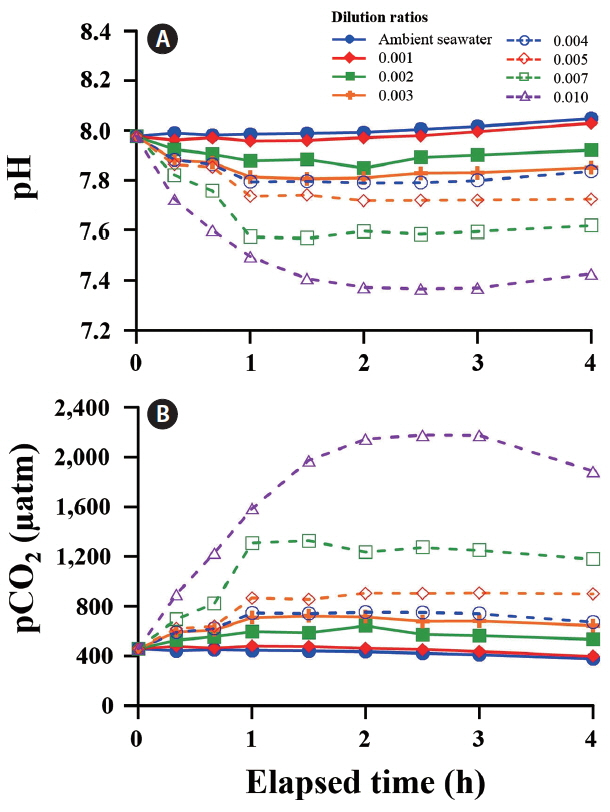
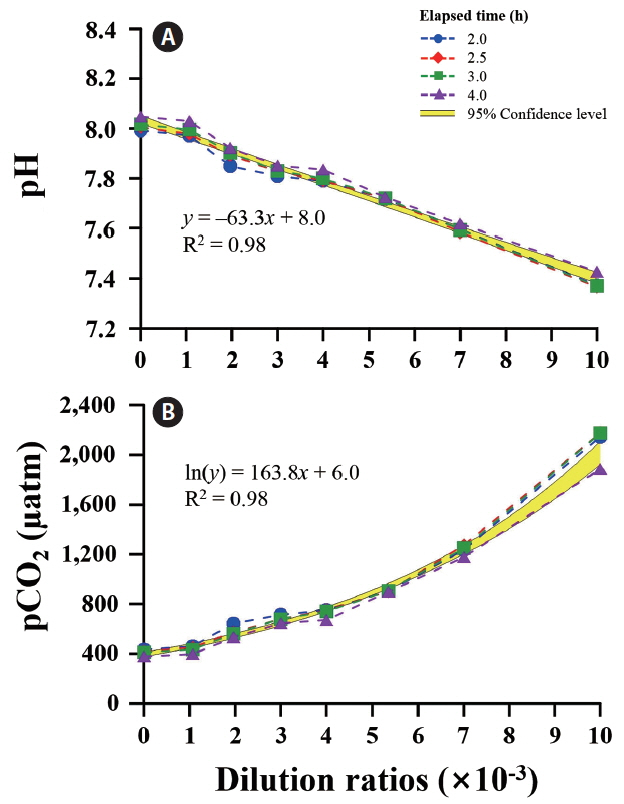
A wide range of pH and pCO2 can be controlled by adjusting the dilution ratios between ambient and CO2-saturated seawaters. After adding CO2-saturated seawater to the tanks, initial pH (7.98) and pCO2 (452 μatm) of ambient seawater changed rapidly and reached a steady state level of pH 7.30 and pCO2 ca. 2,600 μatm within 4 h (Fig. 3). In all tanks, CO2-induced changes in carbonate chemistry achieved a plateau level of 80% after 1 h. A significant negative correlation was observed between pH and the dilution ratio of CO2-saturated to ambient seawaters (R2 = 0.98). The pCO2 increased exponentially with increasing dilution ratio (Fig. 4).
Daily fluctuation of carbonate chemistry by dilution ratio
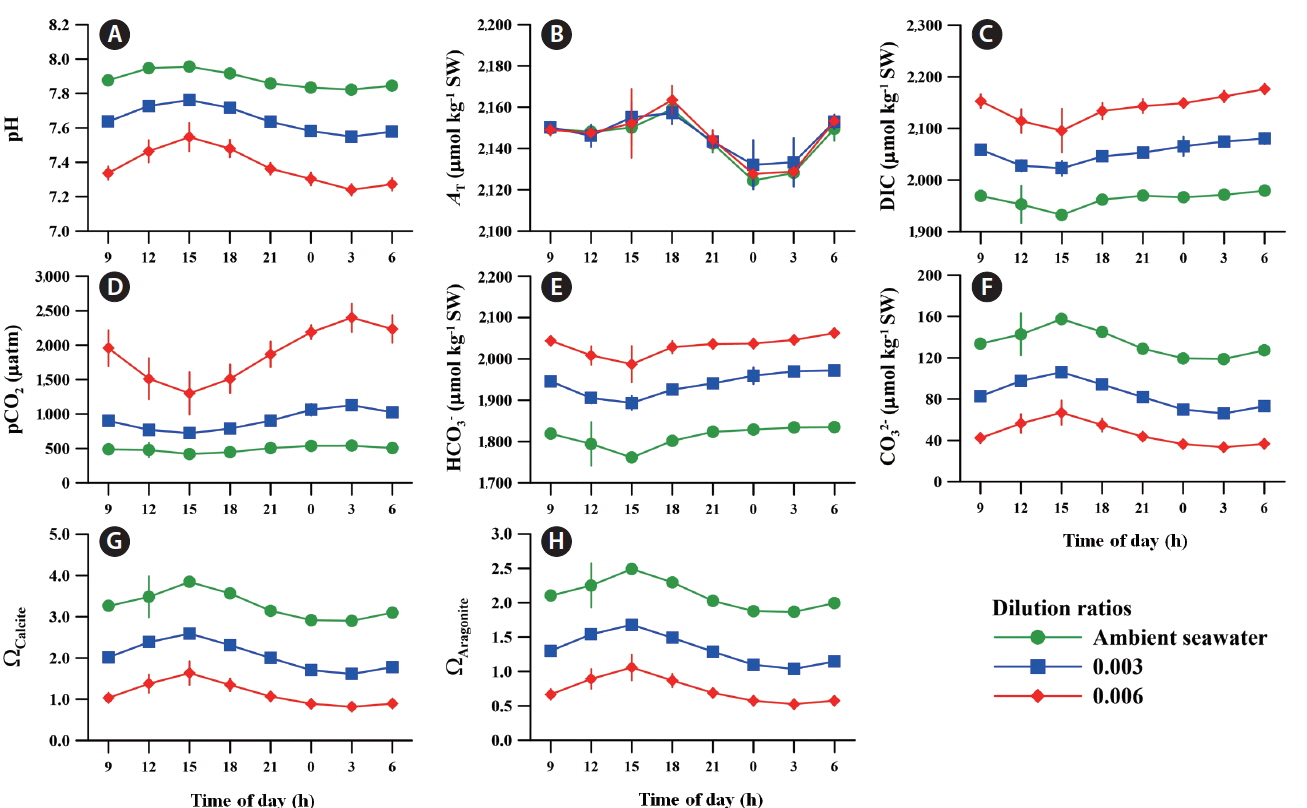
In general, the trend in carbonate chemistry in both ambient and acidified seawaters is close to a cosine curve over the time of day, regardless of the light and dark cycle (Fig. 5). The pH and carbonate ion (CO32−) values gradually increased with increasing irradiance during the daytime (from 9:00 am to 3:00 pm), whereas dissolved inorganic carbon (DIC), pCO2, and bicarbonate (HCO3−) values decreased. The highest pH of 7.96 ± 0.01 was recorded on the same day that the irradiance reached its peak power at 3:00 pm, while its value was 7.88 ± 0.01 at the beginning (9:00 am) (Fig. 5A). The pCO2 value of ambient seawater decreased from 487 ± 8 to 419 ± 2 μatm during this period (Fig. 5D).
The decline in pH and increase in pCO2 occurred from 3:00 pm to 3:00 am, which correlates with the fact that respiration exceeds photosynthesis as irradiance decreased. During these hours, it reached the lowest pH and the highest pCO2 values at 3:00 am in ambient conditions. Specifically, the fluctuations of DIC, HCO3−, and CO32− in ambient seawater demonstrated a countertrend between the hours of day and night.
The pCO2 values in the OA conditions with dilution ratios of 0.003 and 0.006 were 84% (899 ± 29 μatm) and 300% (1,957 ± 261 μatm) higher than that in ambient conditions. The pH and pCO2 levels in acidified seawater followed the same patterns in ambient seawater and exhibited a larger amplitude in daily fluctuations than in ambient conditions. Other carbonate chemical parameters including HCO3−, CO32−, DIC, calcium carbonate state (ΩCalcite), and aragonite saturation state (ΩAragonite) in OA conditions also fluctuated in parallel with those of ambient conditions. The AT varied slightly from 2,214 ± 2 to 2,159 ± 0 μmol kg−1 SW during the day despite significant level changes in other carbonate parameters over time (Fig. 5B).
DISCUSSION
Research on OA has increased rapidly in recent years with growing concerns about climate change. We designed a cost efficient mesocosm facility for an OA experiment that applies an outdoor continuous flow-through tank system. Generally, a benthic mesocosm requires a higher degree of specificity for its experimental setup to appropriately reflect different target populations or communities. There are various mesocosm approaches suggested for the ecological evaluation of OA on marine benthic organisms; however, many facilities are required to resolve the associated problems including high costs and degree of reality achieved. In previous studies, the elevated CO2 conditions were often aerated with the premixed CO2 gas (Alexandre et al. 2012, Pansch et al. 2016), and they required a large amount of mixed gases to manipulate OA conditions, in addition to being expensive (more than US $200 per 50 L gas cylinder).
Andersson et al. (2009) designed a continuous flow-through system that was adjusted with weak HCl to maintain elevated CO2 conditions. However, this method causes a fractional shift in the seawater’s carbonate chemistry, which is dissimilar to natural OA conditions. For example, a greater fractionation of DIC (HCO3− and CO32−) shifts towards more CO2, and this process can change the total alkalinity of seawater while DIC remains unchanged. Alternatively, the compressed CO2 gas flowed into the submerged water pump with a gas control valve to create high CO2 conditions, and this system was applied to a natural seagrass habitat (Campbell and Fourqurean 2011). Another low-cost tank system was implemented to regulate CO2 inflow with a peristaltic pump that controlled CO2 flow and a microfine bubble diffuser (Jokiel et al. 2014).
In this study, we have suggested a novel mesocosm that was adopted on-site using low-cost and easy-to-use platforms to control CO2 concentration. Seawater carbonate chemistry was adjusted by adding calculated quantities of CO2-saturated seawater to ambient seawater, for which the DIC was known and had a similar shift in all tanks that corresponded with the desired treatments. The addition of CO2-saturated seawater is a more effective method for creating acidified seawater, as compared to methods involving CO2 bubbling and adding weak HCl (Kim et al. 2010, 2016, Riebesell et al. 2010). This technique has not been previously applied to OA research for marine benthos. It is considered one of the most efficient methods that allows for the carbonate chemistry to quickly reach the target value from the ambient value. This pumping system can achieve the desired CO2 levels quickly with a short seawater residence time of approximately 2.5 h in the tank, and it can realize the target level of 80% in 1 h. Another important point is that the dilution ratio of CO2-saturated seawater to ambient seawater can be readily applied for running OA experiments in the field. The addition of CO2-saturated seawater was regulated with a high-precision peristaltic pump that controlled the flow rate in a range of 0–85 mL min−1 and kept the flow rate constant for a given period of time. An additional advantage is that the use of a continuous flow-through systems allows for pulsed exposure to be more realistically simulated with diel fluctuations in seawater carbonate chemistry, as well as other environmental factors, during the experiment.
Marine benthic autotrophs (e.g., macroalgae and seagrass) experience dynamic changes in CO2 and pH in respect to photosynthesis and respiration (Jiang et al. 2011, Kim et al. 2015). The magnitude of pCO2 fluctuations is greater in coastal areas (e.g., upwelling, estuary, near shore, coral reef, and kelp forests) than in the open ocean, and are mainly determined by biological processes (Hofmann et al. 2011). Variations of pCO2 in seagrass beds ranged from 240 to 1,488 μatm, representing changes in biological activity with daily or seasonal fluctuations (Saderne et al. 2013). Additionally, pCO2 survey in a giant kelp forest identified a strong relationship with that of surrounded seawater (Frieder et al. 2012). Because our mesocosm is designed to produce rapid mixing and is subject to natural fluctuations in pCO2, it is useful to explore the biological consequences of OA in more realistic field conditions.
As pH decreased, the pCO2 increased exponentially, which is expected under climate change scenarios (Fig. 4). Diel fluctuations of pCO2 was large at elevated CO2 conditions; however, pH was lower and changed in parallel with ambient conditions (Fig. 5). Generally, seawater pCO2 increased exponentially at the pH range from the pre-industrial revolution (approximately 8.2) to future climate scenarios, which is predicted to decrease pH by 0.5 units by 2,100 (Raven et al. 2005). The changes in carbonate chemistry speciation in mesocosm tanks are largely induced by intensive biogeochemical process such as photosynthesis, respiration, and decomposition of marine biota in various ways. From these aspects, our field mesocosm is suitable for fulfilling ecological requirements and can be used for gauging responses of coastal ecosystems where the dynamics of carbonate chemistry are mainly affected by biological processes.
All facilities can be supplied at a reasonable price (e.g., small water tank, pure CO2 gas, and peristaltic pump), which is a major advantage over the other techniques previously used for outdoor mesocosm studies. Moreover, this technique has a fast response time and it is feasible for manipulating carbonate chemistry of seawater to reach desired levels of pCO2 and total alkalinity. However, high quality carbonate chemistry measurements are required in order to fully understand the dynamics of a carbonate system in ambient and acidified treatments. In order to satisfy this requirement, the precision of seawater pH measurements must be better than 0.002 pH units (Rérolle et al. 2012). In this study, the dilution method using CO2-saturated seawater highlighted the cost efficiency and technical feasibility for creating desired CO2 conditions, as well as the advantage of representing the fluctuations in carbonate chemistry for various coastal environments.