ABSTRACT
Ecklonia cava is popular in Korea as a marine functional materials. E. cava is generally collected and used on the coast of Jeju Island. However, the continuous use of collected natural E. cava may be limited because difficult to secure throughout the year and may be exposed to environmental pollution. Jeju magma seawater (MSW) was known to be significant advantages such as safety, cleanness, stability, and functional improvement. Attempts have been reported on application of MSW to the culturing of macro- and microalgae and showed improved results. Thus, the objective of the present study was to explore the anti-melanogenesis activity of brown seaweed E. cava (E. cava cultured with MSW [MSWE]) extract cultured in tanks with MSW of Jeju Island to evaluate the possibility of cosmeceutical industrial application. MSWE extract showed the higher polyphenolic and dieckol contents than natural E. cava (NE) extract. Anti-melanogenesis activity of MSWE extract and NE extract are tested and compared using tyrosinase and dihydroxyphenylalanine (DOPA) oxidation inhibition assay. MSWE extracts evidenced more effective tyrosinase and DOPA oxidation inhibition activity than that of the NE extracts and the commercial whitening agent, arbutin. MSWE extracts also markedly inhibited melanin synthesis and decreased the expression of melanogenesis-related protein in α-melanocyte stimulating hormone-stimulated B16F10 melanoma cells without cytotoxicity. These results suggest that MSW cultivation process would be more effective in releasing bioactive compounds with whitening effect from seaweed such as E. cava at an industrial scale.
INTRODUCTIONSeaweeds are rich in minerals, dietary fibers, proteins, polysaccharides and various marine polyphenols (Choi et al. 2016, Fernando et al. 2017). Ecklonia cava is a brown seaweed that is abundant in the subtidal regions of Jeju Island in Korea. E. cava has been identified as a potential producer of wide spectrum of natural substances such as carotenoids, fucoidans, and phlorotannins, that show different biological activities (Wijesinghe and Jeon 2012). Notably, phlorotannins components and/or extract of E. cava are potential cosmetic and cosmeceutical agents because of their biological activities (Lee et al. 2015, Sanjeewa et al. 2016). Thus, E. cava is popular and important in Korea as a marine functional materials for commercial use. E. cava is generally collected and used on the coast of Jeju Island. However, the continuous use of collected natural E. cava may be limited because difficult to secure throughout the year and may be exposed to environmental pollution. In addition, recent several studies have reported the seasonal variations in bioactive components in brown seaweed species as a function of season and collected location (Terasaki et al. 2009, 2012, Nomura et al. 2013). Against this backdrop, it is necessary to attention for develop sustainable resources of seaweed that can be used in the development of innovative cosmeceutical and pharmaceutical ingredients.
In recent years, various methods such as cultivated in artificial seawater prepared with simplified recipes and deep seawater (DSW) have been proposed to achieve sustainability and maintaining seaweeds of commercial importance under laboratory conditions (Kaladharan 2000, Mori et al. 2004, Hiraoka and Oka 2008, Msuya and Neori 2008). At present, seaweed cultivation technologies have developed dramatically in Asia to improve industrial use (Kim et al. 2017). Attempts have been reported on application of DSW to the tank cultivation of seaweed and showed improved results such as increasing daily growth rate and bioactive substances content (Mori et al. 2004, Hiraoka and Oka 2008). Magma seawater (MSW) is underground seawater produced from region, located at northeast part of Jeju Island and has several characteristic properties such as abundance of mineral contents, cleanness (pathogen- and pollution-free), low temperature throughout the year, and biological activities (Noh et al. 2010, Bae et al. 2012). These characteristics make MSW suitable for tank mass cultivation of seaweeds. However, a stable tank mass culture of seaweeds using MSW and bioactive properties of cultured seaweeds has not been studied.
Thus, the objective of the present study was to explore the anti-melanogenesis activity of brown seaweed E. cava extract cultured in tanks with MSW of Jeju Island to evaluate the possibility of cosmeceutical industrial application.
MATERIALS AND METHODSMaterialsNatural E. cava (NE) and E. cava cultured with MSW (MSWE) were supplied from the Jeju International Marine Science Research & Education Center of Korea Institute of Ocean Science & Technology (KIOST). The natural E. cava was collected from the intertidal area of Sungsan beach in Jeju Island. The natural E. cava was submersed in 10 ton-scaled 5 tanks filled with Jeju magma seawater (JMS, underground seawater from 150 m depth from tube well on the ground) and was cultured for 15–30 days with whole change of JMS in every day. Then, the both samples were rinsed carefully with fresh water and freeze-dried. Dried samples were ground and sifted through a 50-mesh standard testing sieve. All chemicals and reagents used were of analytical and obtained from commercial sources.
Preparation of the MSWE extractsThe ground E. cava cultured with MSW powder (1 g) was mixed with 100 mL of water and ethanol (50% and 70% concentration each) and placed in a shaking incubator for 24 h at room temperature. The extracts were centrifuged at 3,500 rpm for 20 min at 4°C and filtered through Whatman filter paper (Whatman, Maidstone, UK) to remove the residue. Next, the filtrate were evaporated under vacuum at 40°C to remove ethanol. All samples were kept at −20°C until further use. In this study, tyrosinase inhibitory effects of all the extracts from MSWE are tested and compared. The concentration of sample producing 50% inhibition of the tyrosinase (IC50) was used as an index. Fifty percent ethanol extracts showed lower IC50 values (22.80 μg mL−1) than 70% ethanol (43.32 μg mL−1) extracts and water extracts (>100 μg mL−1). As 50% ethanol extracts possessed the highest tyrosinase inhibitory effect, this extract was selected for further analysis and experiments.
Preparation of the NE extractsThe ground NE powder (1 g) was mixed with 100 mL of 50% ethanol and placed in a shaking incubator for 24 h at room temperature. The extracts were centrifuged at 3,500 rpm for 20 min at 4°C and filtered through Whatman filter paper to remove the residue. Next, the filtrate were evaporated under vacuum at 40°C to remove ethanol. All samples were kept at −20°C until further use.
Determination of the total polyphenolic, carbohydrate, and protein contentsPhenolic content was determined according to a protocol similar to that of Chandler and Dodds (1983). Each 1 mL of E. cava extracts, 1 mL of 95% EtOH, 5 mL of distilled water, and 0.5 mL of 50% Folin-Ciocalteu reagent were mixed. The mixtures were allowed to react for 5 min, and then 1 mL of 5% Na2CO3 was added, and the mixture was thoroughly mixed and placed in the dark for 1 h. Absorbance was measured at 725 nm and gallic acid standard curve was obtained for the calibration of phenolic content. The carbohydrate content was determined by the phenol-sulfuric acid method using glucose as a standard. The protein content was determined by using a commercial assay kit (BCA Protein Assay Kit; Thermo Fisher Scientific, Waltham, MA, USA).
Assay for the measurement of inhibitory effects on mushroom tyrosinaseMushroom was used as the source of tyrosine for the entire study. Each extract (10 μL) was diluted with 110 μL of 0.1 M sodium phosphate buffer (pH 6.8) in a 96-well plate and then 10 μL of mushroom tyrosinase (1,500–2,000 units) and 20 μL of 1.5 mM L-tyrosine solution were added into a 96-well plate. The test mixture was mixed well and incubated at 37°C for 12 min.
Following incubation, the amount of dopachrome produced in the reaction mixture was determined at 490 nm using microplate reader.
Assay for the measurement of inhibitory effects on dihydroxyphenylalanine oxidationThe dihydroxyphenylalanine (DOPA) oxidation inhibitory activity of mushroom tyrosinase was measured using a colorimetric assay, which relies on the oxidation of DOPA to DOPA-quinone. Each extract (10 μL) was diluted with 170 μL of 0.1 M Tris-HCl buffer (pH 6.8) in a 96-well plate and then 10 μL of mushroom tyrosinase (1,000 units) and 10 μL of 10 mM DOPA solution were added into a 96-well plate. The test mixture was mixed well and incubated at 37°C for 15 min. Following incubation, the amount of dopachrome produced in the reaction mixture was determined at 490 nm using microplate reader.
Analysis of quantity of dieckolThe high-performance liquid chromatography (HPLC) system (Waters 2998; Waters, Milford, MA, USA) comprising the following components: photodiode array detector, gradient pump, vacuum degasser, and mixer were used for the determination of quantity of dieckol. The column used a RP-C18 column (250 × 4.6 mm, 5 μM; Waters). For HPLC analysis, mobile phases used in the gradient elution consisted of primary eluant (A) consisting of distilled water, and a secondary eluant (B) consisting of acetonitrile. Ninety-five percent solvent A changed, in the linear gradient, to 100% of B 50 min after injection. The flow rate was 1 mL min−1, the column temperature was a room temperature (20°C), and sample volume injected was 20 μL. The absorbance was measured at a wavelength 230 nm for the detection of dieckol.
Cell cultureB16F10 cells obtained from the Korea Cell Line Bank were grown in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, penicillin (100 U mL−1) and streptomycin (100 μg mL−1). Cells were maintained at 37°C in a 5% CO2 incubator. B16F10 cells were cultured in 24-well plates for melanin quantification and enzyme activity assays.
Cell viabilityCell viability was quantified through a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which measures mitochondrial activity in viable cells. B16F10 cells were seeded (5 × 104 cells mL−1) with various concentrations of samples and incubated for up to 72 h prior to MTT treatment. An MTT stock solution (50 μL; 2 mg mL−1 in phosphate buffered saline [PBS]) was added to each well to achieve a total reaction volume of 250 μL. After 4 h of incubation, the plates were centrifuged for 10 min at 2,000 rpm, and the supernatants were aspirated. The formazan crystals in each well were dissolved in dimethyl sulfoxide. The amount of purple formazan was assessed by measuring the absorbance at 540 nm.
Measurement of cellular melanin contentsCellular melanin content was determined as previously described, with modifications (Bilodeau et al. 2001). The amount of melanin was used as an index for melanogenesis. B16F10 cells (5 × 104 cells mL−1) were seeded in 24-well dishes and incubated in the presence or absence of 0.1 μM α-melanocyte stimulating hormone (α-MSH). The cells were then incubated for 72 h with various concentrations of samples. The samples were washed with PBS and dissolved in 100 μL of 1 N NaOH. The samples were incubated at 60°C for 1 h and mixed to solubilize the melanin. The amount of melanin was assessed by measuring the absorbance at 405 nm.
Western blot analysisB16F10 cells were treated with the indicated concentrations of samples and harvested. The cell lysates were prepared with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, and 1 mM ethylenediaminetetraacetic acid). Cell lysates were cleared via centrifugation, and the protein concentration was determined using a bicinchoninic acid assay protein assay kit. Proteins (30 μg) were subjected to electrophoresis on 10 or 12% sodium dodecyl sulfate–polyacrylamide gels, and were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated with primary antibodies against tyrosinase, microphthalmia-associated transcription factor (MITF), tyrosinase-related protein (TRP) 1, TRP2, and β-actin in TTBS (25 mM Tris-HCl, 137 mM NaCl, 0.1% Tween 20, pH 7.4) containing 0.5% nonfat dry milk for 1 h. The membranes were then washed with TTBS and incubated with secondary antibodies. Signals were developed using an enhanced chemiluminescence Western blotting detection kit and exposed to X-ray films.
RESULTSExtraction yield, total polyphenolic, carbohydrate, and protein contents as well as HPLC analysis of dieckolThe extraction yield and total polyphenolic, carbohydrate, and protein contents of extracts obtained from NE and MSWE are shown in Table 1. The NE extracts showed higher extraction yield than the MSWE extracts. Despite the lower extraction yield, MSWE extracts had higher total polyphenolic, carbohydrate, and protein contents than NE extracts. For the analysis of dieckol, the major polyphenol in the E. cava, HPLC analysis carried out. The dieckol analysis of each extract are indicated in the Fig. 1. The HPLC analysis revealed that dieckol was the major polyphenol in the extracts, with minor other polyphenolic compounds. Especially, MSWE extracts showed noticeable amount of dieckol compared to the NE extracts. The dieckol contents of MSWE extracts and NE extracts were 6.80 and 5.09%, respectively. Taken together, these data indicate that cultivation application in MSW for extraction of bioactive components of E. cava may be more advantageous than NE.
Inhibitory effect of MSWE extracts against tyrosinase and DOPA oxidationAnti-melanogenesis activity of the extracts obtained from NE and MSWE was evaluated and compared using tyrosinase and DOPA oxidation inhibition assays. As shown in Fig. 2, MSWE extracts inhibited tyrosinase activity and DOPA oxidation in a dose-dependent manner. Moreover, MSWE extracts evidenced more effective tyrosinase and DOPA oxidation inhibition activity than that of the NE extracts and the commercial whitening agent, arbutin. In particular, IC50 values of MSWE extracts against tyrosinase activity and DOPA oxidation were 22.8 and 34.4 μg mL−1, respectively, which were evidenced stronger inhibitory effect than was observed with NE extracts. These results indicated that noticeable amount of dieckol and minor other polyphenolic compounds in the MSWE extracts may be attributed to the observed anti-melanogenesis activities in this study.
Effects of MSWE extracts on melanin systhesis in α-MSH-stimulated melanoma cellsWe first examined the cytotoxic effect of MSWE extracts on B16F10 melanoma cells treated with various concentrations of MSWE extracts (25–100 μg mL−1). As shown in Fig. 3A, MSWE extracts was not cytotoxic at the concentrations tested. In addition, arbutin and α-MSH also exhibited no cytotoxic effects in B16F10 cells. To verify the inhibitor effect of MSWE extracts on α-MSH-mediated melanogenesis, we determined the quantity of intracellular melanin in the presence of α-MSH. As shown in Fig. 3B, MSWE extracts substantially decreased α-MSH-induced cellular melanin content in a dose-dependent manner, as compared to cells treated with α-MSH alone. Especially, at the same concentrations, MSWE extracts exhibited slightly higher inhibitory effect against melanin synthesis than NE extracts. Moreover, arbutin had a weaker effect on melanin synthesis than MSWE extracts at the same concentrations. These results suggest that MSWE extracts inhibited tyrosinase activity, and that this inhibitory effect may lead to decreased cellular melanin synthesis in B16F10 cells.
Effect of MSWE extracts on tyrosinase, TRP-1, TRP-2, and MITF expression in α-MSH-stimulated melanoma cellsIn order to explore the mechanisms underlying MSWE extracts-mediated inhibition of melanin synthesis, we determined the effects of MSWE extracts on the expression level of signaling molecules involved in melanin synthesis. B16F10 cells were exposed to MSWE extracts in the presence of α-MSH, and changes in the expression of tyrosinase, TRP-1, TRP-2, and MITF were analyzed by western blot analysis. As shown in Fig. 4, MSWE extracts effectively reduced α-MSH-induced expression of tyrosinase, TRP1, TRP2, and MITF and it had a greater effect than arbutin on protein expression. These results suggest that MSWE extracts inhibited tyrosinase activity and melanin synthesis through downregulation of TRP expression.
DISCUSSIONMSW and DSW is attracting biological and industrial interest because of following advantages: its temperature constantly low throughout the year; it is clean; and it contains a higher concentration of major inorganic nutrients (Mori et al. 2004, Noh et al. 2010, Bae et al. 2012). These characteristics make MSW and MSW suitable for tank mass cultivation of seaweeds. Actually, DSW has been used for experimental tank cultivation of seaweeds (Mori et al. 2004, Hiraoka and Oka 2008). Our research group has recently started a new project that aims to use MSW as a cultivation medium for seaweed based on the above-mentioned merits. E. cava contains a variety of bioactive compounds, including carotenoids, fucoidan, and phlorotannins, that show different biological activities (Lee et al. 2015). For this reason, E. cava has been cited as a good candidate for the source of natural functional materials. However, a stable tank mass culture of E. cava using MSW and bioactive properties of cultured E. cava has not been studied. Thus, the present study E. cava extracts cultured in tanks with MSW of Jeju Island was first investigated for their extraction yield, total polyphenolic content, and anti-melanogenesis activities. Further, our aim also was to use this strategy to develop sustainable resources of bioactive compounds that can be used in the development of pharmaceutical and cosmeceutical ingredients.
Phenolic compounds are one of the most interesting water soluble plant substances naturally present in cell vacuole, which are carrying numerous biological activities. Within the last few decades, many researchers have reported that polyphenolic compounds are believed as the major bioactive compounds found in biological systems (Handique and Baruah 2002). E. cava contains biologically active compounds such as polyphenols called phlorotannins eckol, 6,6′-bieckol, dieckol, and triphlorethol-A (Heo et al. 2009). Among these phlorotannins, dieckol is one of the major and active compounds. It have shown interesting functional and bioactive properties (Wijesinghe and Jeon 2012, Sanjeewa et al. 2016). The present results showed that although MSWE extract exhibited the lower extraction yield than NE extract, MSWE extract had higher total polyphenolic content and dieckol content than NE extract. These findings suggest that polyphenolic content and dieckol content of E. cava were increased by the cultivation in MSW, comparing to original E. cava and might induce increased biological activities. Recently, Bae et al. (2012) reported that the in vitro cultivation of Bletilla striata using Jeju MSW increased chlorophyll contents, compared with non-cultured Bletilla striata.
E. cava is popular in Korea as a cosmetic ingredient and marine herb due to the wide range of biological activities associated with natural compounds of E. cava. Previous studies have proposed that E. cava can be explored as a potential whitening agent. For example, E. cava extracts has profound inhibitory effects against tyrosinase and melanin systhesis (Cha et al. 2011). Another study indicated that dieckol isolated from E. cava has strong de-pigmenting activity without discernible cytotoxicity in B16F10 melanoma cells (Heo et al. 2009, Kang et al. 2012). Further to these results, we wanted to elucidate the biological effects of E. cava extracts cultured in MSW and therefore investigated the identical anti-melanogenesis effect in the present study. Tyrosinase has long been known to be essential for melanization. In vertebrates, tyrosinase, the enzyme responsible for the initial steps of melanin synthesis, is closely associated with specialized organelles called melanosomes, which are found in melanocytes (Chakraborty and Chakraborty 1993). Many commercial tyrosinase inhibitors are used in cosmetics and medical applications to treat skin and pigment abnormalities. This present study showed that all tested samples has strong tyrosinase and DOPA oxidation inhibitory effects. We determined that among the tested extracts, MSWE extract exhibited greatest tyrosinase and DOPA oxidation inhibitory effects. It has been reported that the presence of a hydroxyl group and of an electron donator group in the phenol ring is a primary requirement to effectively serve as a tyrosinase substrate (Fenoll et al. 2000). Thus, these results suggest that MSWE extract was a more effective inhibitor of tyrosinase than comparing to NE extract due to higher polyphenolic content and dieckol content. Accordingly, the increase in tyrosinase inhibition activity suggest that MSWE extract may enhance inhibition of melanin synthesis.
Melanin production correlates directly with the activity of tyrosinase and the protein levels of tyrosinase (Maeda et al. 1997). As MSWE extract possessed the highest tyrosinase inhibition activity; this extract was further investigated for its inhibiting effect melanin synthesis in the presence of α-MSH. We identified that MSWE extract inhibited melanin synthesis and protein levels of tyrosinase in a dose-dependent manner, which was not a cytotoxic effect to B16F10 cells. In addition, MSWE extract confirmed comparatively better inhibiting melanin synthesis than NE extract. Accordingly, the present study suggests that MSWE extract may a more effective downregulate tyrosinase activity and inhibit cellular melanin synthesis in B16F10 cells. In general, α-MSH potently induces MITF expression, which is stimulated at the levels of tyrosinase, TRP-1, and TRP-2, and which increase melanin synthesis (Kameyama et al. 1995, Bertolotto et al 1998). Thus, we next evaluated the effect of MSWE extract on the expression of MITF, TRP1, and TRP2 to verify the inhibitory effect MSWE extract on melanogenesis. We confirmed that MSWE extract significantly suppressed MITF, TRP1, and TRP2 expression in α-MSH-stimulated B16F10 cells. These results suggest that MSWE extract does directly inhibit tyrosinase activity, as well as that 50% ethanolic extract of MSWE inhibits melanogenesis by suppressing MITF, TRP1, and TRP2 expression in B16F10 cells.
In conclusion, we showed for the first time that MSWE extract has potential to improve anti-melanogenesis activity. We confirmed that MSWE extract exerts beneficial effects on melanogenesis as well as increases polyphenolic content and dieckol content. These results suggest that MSW cultivation process would be more effective in releasing bioactive compounds with whitening effect from seaweed such as E. cava at an industrial scale. Importantly, MSWE extract could be used as a potential natural cosmetics and cosmeceutical source that has potent anti-melanogenesis activity.
ACKNOWLEDGEMENTSThis research was supported by a research grants from the Korea Institute of Ocean Science and Technology (PE99722) and was supported by the Soonchunhyang University Research Fund.
Fig. 1The high-performance liquid chromatography of dieckol analysis of extracts obtained from natural Ecklonia cava (A) and E. cava cultured with magma seawater (B). 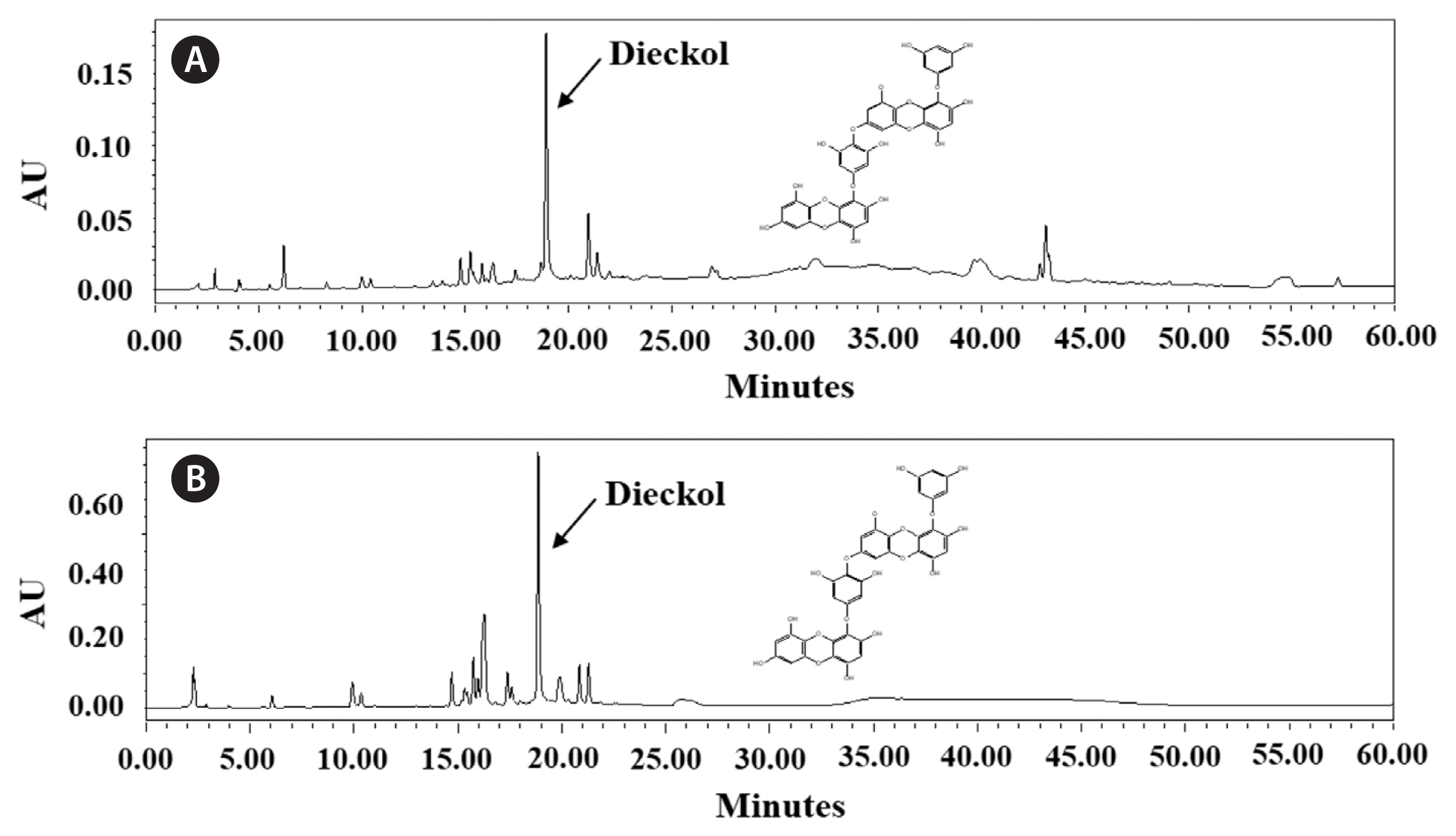
Fig. 2Inhibitory effect of Ecklonia cava cultured with magma seawater (MSWE) and natural E. cava (NE) extracts against tyrosinase (A) and dihydroxyphenylalanine (DOPA) oxidation (B). Arbutin was employed as a positive control. The final concentration of arbutin is 100 μg mL−1. aIC50 value is the concentration of sample required for 50% inhibition. The values are expressed as the mean ± standard error in triplicate experiments. *p < 0.05 indicates a significant difference compared to the NE extract group. 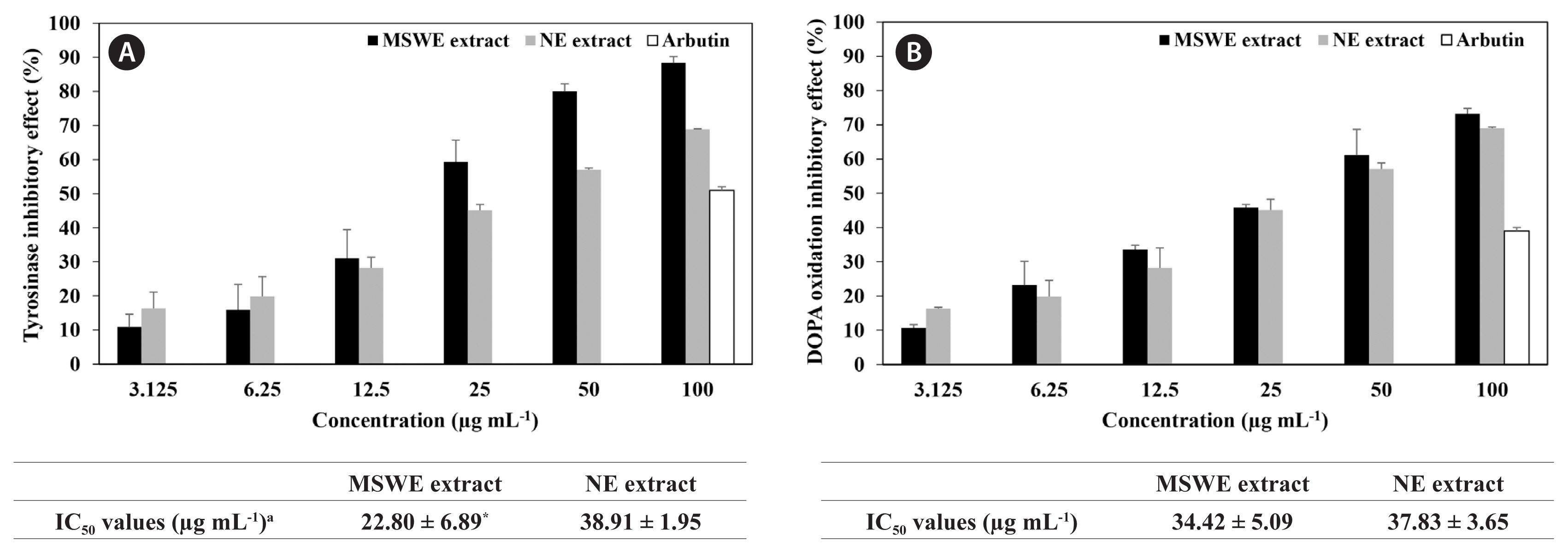
Fig. 3Effect of Ecklonia cava cultured with magma seawater (MSWE) and natural E. cava (NE) extracts on cell viability (A) and cellular melanin synthesis (B) in B16F10 cells. Cells were exposed to 0.1 μM α-melanocyte stimulating hormone (α-MSH) in the presence of the indicated concentrations of extracts or 100 μg mL−1 arbutin. The values are expressed as the mean ± standard error in triplicate experiments. *p < 0.05 indicates a significant difference compared to the NE extract group at the same concentrations. 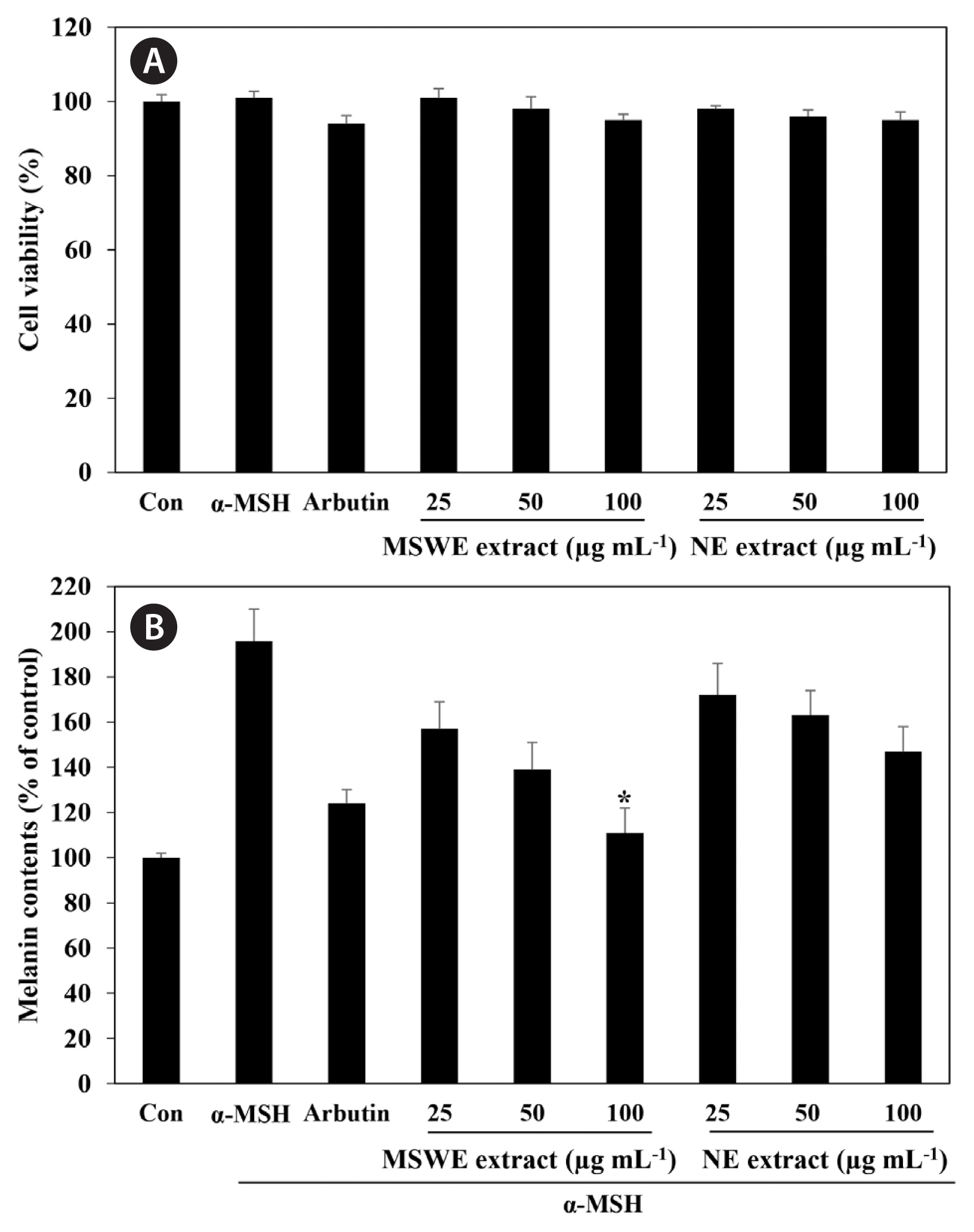
Fig. 4Effect of Ecklonia cava cultured with magma seawater (MSWE) extract on expression of melanogenesis-related proteins in B16F10 cells. Cells were exposed to 0.1 μM α-melanocyte stimulating hormone (α-MSH) in the presence of the indicated concentrations of extracts or 100 μg mL−1 arbutin. Western blot data shown are representative of three independent Western blotting experiments. (A) Microphthalmia-associated transcription factor (MITF), tyrosinase, tyrosinase-related protein (TRP) 1, and TRP2 protein expression. (B) Quantification of MITF, tyrosinase, TRP1, and TRP2 expression. The values are expressed as the mean ± standard error in triplicate experiments. *p < 0.05 indicates a significant difference compared to the only α-MSH-treated cells. 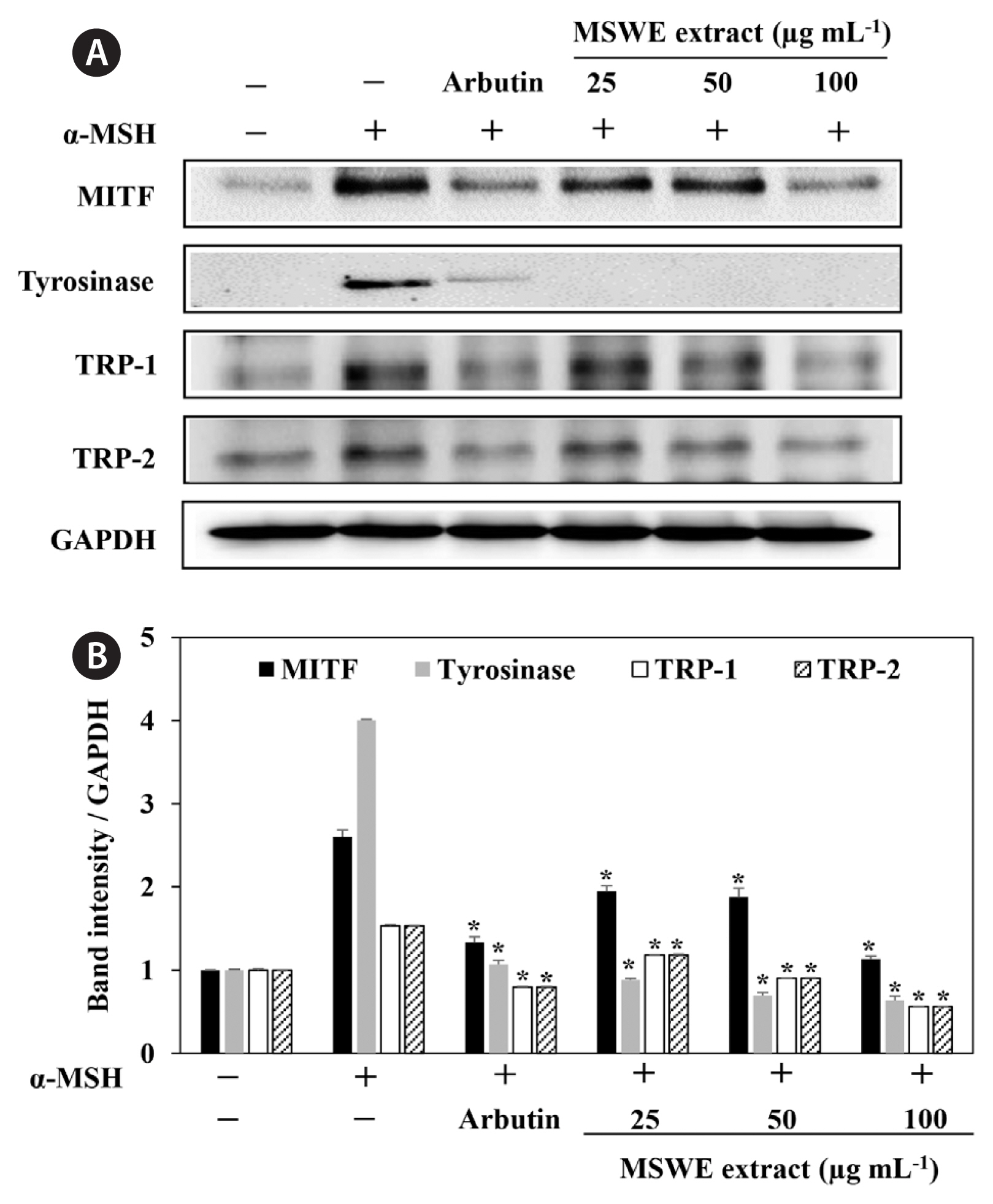
Table 1Comparison of extraction yield and chemical composition of extracts obtained from NE and MSWE REFERENCESBae, K., Kim, KJ., Kim, NY. & Song, JM. 2012. In vitro culture of rare plant Bletilla striata using Jeju magma seawater. J Plant Biotechnol. 39:281–287.
Bertolotto, C., Abbe, P., Hemesath, TJ., Bille, K., Fisher, DE., Ortonne, J. & Ballotti, R. 1998. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 142:827–835.
Bilodeau, ML., Greulich, JD., Hullinger, RL., Bertolotto, C., Ballotti, R. & Andrisani, OM. 2001. BMP-2 stimulates tyrosinase gene expression and melanogenesis in differentiated melanocytes. Pigment Cell Res. 14:328–336.
Cha, S., Ko, S., Kim, D. & Jeon, Y-J. 2011. Screening of marine algae for potential tyrosinase inhibitor: those inhibitors reduced tyrosinase activity and melanin systhesis in zebrafish. J Dermatol. 38:354–363.
Chakraborty, AK. & Chakraborty, DP. 1993. The effect of tryptophan on dopa-oxidation by melanosomal tyrosinase. Int J Biochem. 25:1277–1280.
Chandler, SF. & Dodds, JH. 1983. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep. 2:205–208.
Choi, W., Reid, SNS., Ryu, J., Kim, Y., Jo, Y. & Jeon, BH. 2016. Effects of γ-aminobutyric acid-enriched fermented sea tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae. 31:175–187.
Fenoll, LG., Rodríguez-López, JN., Varón, R., García-Ruiz, PA., García-Cánovas, F. & Tudela, J. 2000. Action mechanism of tyrosinase on meta- and para-hydroxylated monophenols. Biol Chem. 381:313–320.
Fernando, IPS., Kim, H., Sanjeewa, KKA., Oh, J., Jeon, Y. & Lee, WW. 2017. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macrophages by diphlorethohydroxycarmalol isolated from a brown alga Ishige okamurae. Algae. 32:261–273.
Handique, JG. & Baruah, JB. 2002. Polyphenolic compounds: an overview. React Funct Polym. 52:163–188.
Heo, S-J., Ko, S-C., Cha, S-H., Kang, D-H., Park, H-S., Choi, Y-U., Kim, D., Jung, W-K. & Jeon, Y-J. 2009. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro. 23:1123–1130.
Hiraoka, M. & Oka, N. 2008. Tank cultivation of Ulva prolifera in deep seawater using a new “germling cluster” method. J Appl Phycol. 20:97–102.
Kaladharan, P. 2000. Artificial seawater for seaweed culture. Indian J Fish. 47:257–259.
Kameyama, K., Sakai, C., Kuge, S., Nishiyama, S., Tomita, Y., Ito, S., Wakamatsu, K. & Hearing, VJ. 1995. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell Res. 8:97–104.
Kang, S., Heo, S., Kim, K., Lee, S., Yang, H., Kim, A. & Jeon, Y-J. 2012. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg Med Chem. 20:311–316.
Kim, JK., Yarish, C., Hwang, EK., Park, M. & Kim, Y. 2017. Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae. 32:1–13.
Lee, S-H., Kang, S-M., Sok, CH., Hong, JT., Oh, J-Y. & Jeon, Y-J. 2015. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae. 30:163–170.
Maeda, K., Yokokawa, Y., Hatao, M., Naganuma, M. & Tomita, Y. 1997. Comparison of the melanogenesis in human black and light brown melanocytes. J Dermatol Sci. 14:199–206.
Mori, K., Ooi, T., Hiraoka, M., Oka, N., Hamada, H., Tamura, M. & Kusumi, T. 2004. Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar Drugs. 2:63–72.
Msuya, FE. & Neori, A. 2008. Effect of water aeration and nutrient load level on biomass yield, N uptake and protein content of the seaweed Ulva lactuca cultured in seawater tanks. J Appl Phycol. 20:1021–1031.
Noh, J., Gang, G., Kim, Y., Yang, K., Lee, C., Na, O., Kim, G., Oh, W. & Lee, Y-D. 2010. Desalinated underground seawater of Jeju Island (Korea) improves lipid metabolism in mice fed diets containing high fat and increases antioxidant potential in t-BHP treated HepG2 cells. Nutr Res Pract. 4:3–10.
Nomura, M., Kamogawa, H., Susanto, E., Kawagoe, C., Yasui, H., Saga, N., Hosokawa, M. & Miyashita, K. 2013. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the Northern seashore of Japan. J Appl Phycol. 25:1159–1169.
Sanjeewa, KKA., Kim, E., Son, K. & Jeon, Y-J. 2016. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: a review. J Photochem Photobiol B Biol. 162:100–105.
Terasaki, M., Hirose, A., Narayan, B., Baba, Y., Kawagoe, C., Yasui, H., Saga, N., Hosokawa, M. & Miyashita, K. 2009. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J Phycol. 45:974–980.
|
|
|||||||||||||||||||||||||||||||||||||||||