ABSTRACTThe dinoflagellate genus Alexandrium is known to often form harmful algal blooms causing human illness and large-scale mortality of marine organisms. Therefore, the population dynamics of Alexandrium species are of primary concern to scientists and aquaculture farmers. The growth rate of the Alexandrium species is the most important parameter in prediction models and nutrient conditions are critical parameters affecting the growth of phototrophic species. In Korean coastal waters, Alexandrium affine and Alexandrium fraterculus, of similar sizes, often form red-tide patches together. Thus, to understand bloom dynamics of A. affine and A. fraterculus, growth rates and nitrate uptake of each species as a function of nitrate (NO3) concentration at 100 μmol photons m−2 s−1 under 14-h light : 10-h dark and continuous light conditions were determined using a nutrient repletion method. With increasing NO3 concentration, growth rates and NO3 uptake of A. affine or A. fraterculus increased, but became saturated. Under light : dark conditions, the maximum growth rates of A. affine and A. fraterculus were 0.45 and 0.42 d−1, respectively. However, under continuous light conditions, the maximum growth rate of A. affine slightly increased to 0.46 d−1, but that of A. fraterculus largely decreased. Furthermore, the maximum nitrate uptake of A. affine and A. fraterculus under light : dark conditions were 12.9 and 30.1 pM cell−1 d−1, respectively. The maximum nitrate uptake of A. affine under continuous light conditions was 16.4 pM cell−1 d−1. Thus, A. affine and A. fraterculus have similar maximum growth rates at the given NO3 concentration ranges, but they have different maximum nitrate uptake rates. A. affine may have a higher conversion rate of NO3 to body nitrogen than A. fraterculus. Moreover, a longer exposure time to the light may confer an advantage to A. affine over A. fraterculus.
INTRODUCTIONThe dinoflagellate genus Alexandrium often forms red tides or harmful algal blooms, causing human illness and large-scale mortality of marine organisms (Anderson et al. 2012). Therefore, the presence and population dynamics of Alexandrium species are primary concerns to scientists and aquaculture farmers (Dias et al. 2015, Eckford-Soper et al. 2016, Hatfield et al. 2019). The growth rate of a red-tide dinoflagellate species is the most important parameter in prediction models of population dynamics (Jeong et al. 2015). Among 34 officially described Alexandrium species, 6–7 species have been revealed to be mixotrophic species (Jacobson and Anderson 1986, Jeong et al. 2005a, 2005b, 2010, Seong et al. 2006, Yoo et al. 2009, Blossom et al. 2012, Lim et al. 2015, Lee et al. 2016). Both exclusively autotrophic and mixotrophic species need nutrients for their growth, and thus nutrient conditions are critical parameters affecting their growth (Yamamoto and Tarutani 1999, Lim et al. 2006, Maguer et al. 2007, Li et al. 2009, Jauzein et al. 2010, Lee et al. 2019). Thus, to understand the population dynamics of an Alexandrium species and to predict the outbreak of red tides or harmful algal blooms by them, growth rates of the species under different nutrient conditions should be determined.
In Korean coastal waters, Alexandrium affine and Alexandrium fraterculus often co-occur (Lee et al. 1998, Kim 2017, our unpublished data). Both species are known to be potentially toxic and also immobilize and/or lyse other protists (Nguyen-Ngoc 2004, Katsuo et al. 2007, Anderson et al. 2012, Basti et al. 2015, Lee et al. 2016, Kang et al. 2018). One or both of these two species usually form red-tide patches before the outbreak of red tides by the ichthyotoxic dinoflagellate Margalefidinium (Cochlodinium) polykrikoides (Jeong et al. 2017). Thus, the population dynamics of A. affine and/or A. fraterculus can be used for predicting the outbreak of M. polykrikoides red tides. Lee et al. (2016) revealed that A. affine and A. fraterculus lacked mixotrophic ability, whereas Alexandrium andersonii had mixotrophic ability. Therefore, nutrient conditions must be critical parameters affecting their growth. Both A. affine and A. fraterculus are known to have worldwide distributions; A. affine has been observed in European, North American, Asian, and Australian waters, while A. fraterculus has been reported to be in North and South American, Australian and New Zealand, and Japanese waters (Fraga et al. 1989, Nakanishi et al. 1996, Moita and Vilarinho 1999, Band-Schmidt et al. 2003, Hansen et al. 2003, Lagos 2003, MacKenzie et al. 2004, Nguyen-Ngoc 2004, Leaw et al. 2005, Omachi et al. 2007, Lee et al. 2009, Nagai et al. 2009, McCarthy 2013, Park et al. 2013). These waters show a wide range of nutrients such as nitrate and phosphate (Fraga et al. 1989, Nogueira et al. 1997, Kang et al. 2019). Furthermore, many phototrophic dinoflagellates can conduct vertical migration (Jeong et al. 2015); they ascend toward well-lit surface waters during the daytime, but descend toward eutrophic deep waters at night. Theoretically, using swimming speed, A. affine and A. fraterculus could be calculated to travel ca. 15–24 m (Lee et al. 2016, Jeong et al. 2017). Thus, they may experience a wide range of nutrient concentrations every day. To understand the population dynamics of A. affine and A. fraterculus, the effects of nutrient concentrations on their growth rates should be explored. The duration of day or night varies depending on latitudes and season (Van Haren and Compton 2013). The duration of light is known to affect the growth of dinoflagellates and other phytoplankton groups (e.g., Brand and Guillard 1981). Thus, it is worthwhile to explore effects of the duration of daytime or night on growth of A. affine and A. fraterculus.
In this present study, growth rates and nitrate uptake of A. affine and A. fraterculus as a function of nitrate concentration at 100 μmol photons m−2 s−1 under 14-h light : 10-h dark and continuous light conditions were determined using a nutrient repletion method (Lee et al. 2017). The results of the present study provide a basis for understanding ecophysiology of A. affine and A. fraterculus and their bloom dynamics.
MATERIALS AND METHODSPreparation of experimental organisms
A. affine was isolated from coastal waters off Taean (western Korea) in August 2013 when the water temperature and salinity were 21.5°C and 32.2, respectively (Table 1). A. fraterculus was isolated from Yeosu (southern Korea) in September 2013 when the water temperature and salinity were 23.4°C and 32.8, respectively. Clonal cultures for both species were established from two serial single isolations.
A. affine was grown in enriched f/2-Si seawater medium (Guillard and Ryther 1962), while A. fraterculus was grown in enriched L1-Si seawater medium (Guilliard and Hargraves 1993) at 20°C under an illumination of 20 μmol photons m−2 s−1 of cool white fluorescent light on a 14-h light : 10-h dark cycle.
Growth and nitrate uptake rates under light and dark conditionsExperiments 1 and 2 were designed to investigate the growth rates and nitrate (NO3) uptake of A. affine and A. fraterculus as a function of NO3 concentration under light and dark conditions (Table 2). For this measurement, a nutrient repletion method was used (Lee et al. 2017).
Dense cultures of A. affine and A. fraterculus, growing in f/2-Si and L1-Si medium, respectively, were transferred to 800-mL culture flasks. The flasks were placed in a culture room at 20°C under 14-h light : 10-h dark cycle and acclimated at 100 μmol photons m−2 s−1 for 4 d. Three 1-mL aliquots were subsampled and then cells were enumerated to determine the cell concentration. Ten-milliliter aliquots were filtered through GF/F filters (Whatman Inc., Floreham Park, NJ, USA) and then concentrations of NO3 (actually nitrate + nitrite in the Cd-coil reduction method) and phosphate (PO4) were measured using a nutrient analyzer (QuAAtro, Seal Analytical, Norderstedt, Germany).
Cells of either species were added to triplicate 800-mL culture flasks by transferring predetermined volumes of cultures (final cell concentration = ~100 cells mL−1 for both species). A stock solution of NO3 made based on the f/2 medium concentration was added for target final concentrations (NO3 = ca. 110 μM). A sufficient amount of stock solution of PO4 (final concentration = ca. 20 μM), also prepared based on the f/2 medium concentration, was added so that it would not be limiting before NO3 was limiting. Trace metals and vitamins were also added plentifully with consideration of the ratio of nitrate to each chemical in an f/2 medium.
The flasks were placed in a temperature-controlled culture room and incubated at 20°C under an illumination of 100 μmol photons m−2 s−1 of cool white fluorescent light on a 14-h light : 10-h dark cycle. Thirty-milliliter aliquots were subsampled from each flask every day for 2 weeks, and 10-mL aliquots were used for the determination of cell concentration and 20-mL aliquots for the determination of NO3 and PO4 concentrations were filtered through GF/F filters. Cell concentrations (abundances) were determined by enumerating cells on three 1-mL Sedgwick-Rafter counting chambers. The concentrations of nutrients were measured using a nutrient analyzer.
The mean NO3 concentration (N*) at each interval was calculated as:
, where N = NO3 concentration at a single day, t2 − t1 = 1 d.
The specific growth rates of each Alexandrium species (μ, d−1) at each N* were calculated as:
, where Ct1 and Ct2 = cell concentrations of each Alexandrium species at Day t1 and Day t2, respectively.
The maximum growth rate (μmax, d−1) of each Alexandrium species was obtained after data were fitted to a Michaelis-Menten equation:
,where KGR-NO3 = the NO3 concentration sustaining 1/2μmax.
Data were iteratively fitted to the model using DeltaGraph (SPSS Inc., Chicago, IL, USA).
The daily NO3 uptake of each Alexandrium cell was determined by dividing the reduction in N* by the mean cell concentration (C*) at 1 d intervals.
, where t2 − t1 = 1 d.
Day 0 to 3 for A. affine and Day 0 to 1 for A. fraterculus were treated as the acclimation period, and thus data from these days were not used in calculations. The maximum NO3 uptake of each Alexandrium species (V, pM cell−1 d−1) was obtained after data were fitted to a Michaelis-Menten equation;
, where Vmax = maximum uptake rate (pM cell−1 d−1), N* = mean NO3 concentration (μM), and KUT-NO3 = half saturation constant for NO3 uptake (μM).
The mean PO4 concentration (P*), specific growth rates of each Alexandrium species at each P*, and daily PO4 uptake of each cell were calculated in the same manner.
Growth and nitrate uptake rates under continuous light conditionsExperiments 3 and 4 were designed to investigate the growth rates and NO3 uptake of each of A. affine and A. fraterculus as a function of NO3 concentrations under continuous light conditions (Table 2). For this measurement, a nutrient repletion method was used (Lee et al. 2017). The procedure of setting up incubating flasks, subsampling, determining cell abundances and nutrient concentrations, and incubation conditions was the same as in experiments 1 and 2 except for the light conditions.
RESULTSGrowth and nitrate uptake rates of Alexandrium affine under light and dark conditionsIn experiment 1, with increasing incubation time, the concentration of A. affine rapidly increased from 115 cells mL−1 at Day 0 to 5,272 cells mL−1 at Day 10 and then continued to slightly increase (Fig. 1A). The maximum cell concentration (mean ± standard error [SE]) of A. affine concentrations was 7,250 ± 468 cells mL−1 which was achieved at Day 14, the last day of the experiment. The growth rate of A. affine, calculated from linear regression equation, using data between Day 1 and 9, was 0.425 d−1 (Fig. 1B & C). Furthermore, with increasing incubation time, the NO3 concentration (mean ± SE) rapidly decreased from 105.1 ± 0.4 μM at Day 0 to 11.0 ± 0.6 μM at Day 10 and then became 0.9–1.2 μM at Day 11 to 14 (Fig. 1D). Moreover, with increasing incubation time, the PO4 concentration (mean ± SE) rapidly decreased from 21.2 ± 0.3 μM at Day 0 to 4.5 ± 0.2 μM at Day 14 (Fig. 1E). With increasing elapsed incubation time, the ratio of NO3 relative to PO4 decreased from 5.0–5.3 at Day 0–4 to 2.5 at Day 9 and 0.1–0.2 at Day 11–14 (Fig. 1F).
With increasing NO3 concentration, the growth rate of A. affine rapidly increased and then became saturated (Fig. 2A). When the data were fit to Eq. (3), the maximum growth rate (μmax) of A. affine was 0.447 d−1 and the half saturation constant for growth rate (KGR-NO3) was 8.7 μM. However, at the given range of PO4 concentrations, the growth rate of A. affine linearly increased with increasing PO4 concentration (Fig. 2B).
With increasing NO3 concentration, the NO3 uptake of A. affine rapidly increased initially, but then increased more slowly (Fig. 3A). When the data were fit to Eq. (4–6), the maximum NO3 uptake of A. affine was 12.9 pM cell−1 d−1 and the half saturation constant for uptake (KUT-NO3) was 37.5 μM. However, at the given range of PO4 concentrations, the PO4 uptake of A. affine linearly increased with increasing PO4 concentration (Fig. 3B).
Growth and nitrate uptake rates of Alexandrium fraterculus under light and dark conditionsIn experiment 2, with increasing incubation time, the concentration of A. fraterculus increased from 95 cells mL−1 at Day 0 to ca. 4,350 cells mL−1 at Day 13 and then slightly decreased (Fig. 4A). The growth rate of A. fraterculus calculated from linear regression equation using data between Day 1 and 9 was 0.389 d−1 (Fig. 4B & C). Furthermore, with increasing incubation time, the NO3 concentration rapidly decreased from 103.6 ± 0.5 μM at Day 0 to 4.2 ± 2.7 μM at Day 9 and then became 0.8–1.3 μM at Day 10 to 14 (Fig. 4D). Moreover, with increasing incubation time, the PO4 concentration decreased from 21.2 ± 0.2 μM at Day 0 to 11.0 ± 0.2 μM at Day 14 (Fig. 4E). With increasing elapsed incubation time, the ratio of NO3 relative to PO4 decreased from 4.9–5.0 at Day 0–2 to 1.5 at Day 8 and 0.1–0.3 at Day 9–14 (Fig. 4F).
With increasing NO3 concentration, the growth rate of A. fraterculus rapidly increased and then became saturated (Fig. 5A). When the data were fit to Eq. (3), the maximum growth rate (μmax) of A. fraterculus was 0.422 d−1 and half saturation constant for growth rate (KGR-NO3) was 5.4 μM. However, at the given range of PO4 concentrations, the growth rate of A. fraterculus linearly increased with increasing PO4 concentration (Fig. 5B).
With increasing NO3 concentration, the NO3 uptake of A. fraterculus rapidly increased initially, but then increased more slowly (Fig. 6A). When the data were fit to Eq. (4–6), the maximum NO3 uptake by A. fraterculus was 30.1 pM cell−1 d−1 and the half saturation constant for uptake (KUT-NO3) was 44.4 μM. However, at the given range of PO4 concentrations, the PO4 uptake of A. fraterculus linearly increased with increasing PO4 concentration (Fig. 6B).
Growth and nitrate uptake rates of Alexandrium affine under the continuous light conditionsIn experiment 3, with increasing incubation time, the concentration of A. affine rapidly increased from 105 cells mL−1 at Day 0 to 5,779 cells mL−1 at Day 12 and then became saturated (Fig. 7A). The growth rate of A. affine, calculated from a linear regression equation using data between Day 1 and 9, was 0.405 d−1 (Fig. 7B & C). Furthermore, with increasing incubation time, the NO3 concentration rapidly decreased from 110.7 ± 6.3 μM at Day 0 to 15.7 ± 4.2 μM at Day 11 and then became 1.1–2.6 μM at Day 12 to 14 (Fig. 7D). Moreover, with increasing elapsed incubation time, the PO4 concentration decreased from 21.2 ± 0.3 μM at Day 0 to 8.4 ± 0.5 μM at Day 14 (Fig. 7E). With increasing incubation time, the ratio of NO3 relative to PO4 decreased from 5.0–5.2 at Day 0–5 to 1.3 at Day 11 and 0.1–0.3 at Day 12–14 (Fig. 7F).
With increasing NO3 concentration, the growth rate of A. affine rapidly increased and then became saturated (Fig. 8A). When the data were fit to Eq. (3), the maximum growth rate (μmax) of A. affine was 0.458 d−1 and the half saturation constant for growth rate (KGR-NO3) was 21.5 μM. However, at the given range of PO4 concentrations, the growth rate of A. affine linearly increased with increasing PO4 concentration (Fig. 8B).
With increasing NO3 concentration, the NO3 uptake of A. affine rapidly increased initially, but then increased more slowly (Fig. 9A). When the data were fit to Eq. (4–6), the maximum NO3 uptake of A. affine was 16.4 pM cell−1 d−1 and half saturation constant for uptake (KUT-NO3) was 83.3 μM. However, at the given range of PO4 concentrations, the PO4 uptake of A. affine linearly increased with increasing PO4 concentration (Fig. 9B).
Growth and nitrate uptake rates of Alexandrium fraterculus under the continuous light conditionsIn experiment 4, with increasing incubation time, the concentration of A. fraterculus increased from 102 cells mL−1 at Day 0 to 1,587 cells mL−1 at Day 14 (Fig. 10A). The growth rate of A. fraterculus, calculated from linear regression equation using data between Day 2 and 9, was 0.295 d−1 (Fig. 10B & C). Furthermore, with increasing incubation time, the NO3 concentration decreased from 104.3 ± 0.3 μM at Day 0 to 49.9 ± 4.1 μM at Day 14 (Fig. 10D). Moreover, with increasing incubation time, the PO4 concentration rapidly decreased from 21.2 ± 0.3 μM at Day 0 to 17.3 ± 0.5 μM at Day 14 (Fig. 10E). With increasing elapsed incubation time, the ratio of NO3 relative to PO4 decreased from 4.9–5.0 at Day 0–2 to 2.9 at Day 14 (Fig. 10F).
DISCUSSIONThe growth rate of A. affine at 64 μM, which was the mean NO3 concentration between Day 1 and 9, calculated using the Michaelis-Menten type equation in Fig. 2A (0.394 d−1), is only 8% different from that calculated using the linear regression equation in Fig. 1C (0.425 d−1). Similarly, the growth rate of A. fraterculus at 31 μM, which was the mean NO3 concentration between Day 1 and 9, calculated using the Michaelis-Menten type equation in Fig. 5A (0.360 d−1), is also only 8% different from that calculated using the linear regression equation in Fig. 4C (0.389 d−1). Thus, the nutrient repletion method is a reasonable tool for determining growth rates of A. affine and A. fraterculus as a function of NO3 concentration.
The maximum growth rate of A. affine obtained in the present study is comparable to that reported in Nguyen-Ngoc (2004) and Lim et al. (2019), while the maximum growth rate of A. fraterculus obtained in the present study is slightly higher than Lim et al. (2007) (Table 3). The present study reports for the first time the half saturation constant for growth rate (KGR-NO3) and for NO3 uptake (KUT-NO3) of A. affine and A. fraterculus (Table 3). The values of KGR-NO3 and KUT-NO3 are important in understanding competition among red-tide or harmful algal bloom species, because those with lower KGR-NO3 and KUT-NO3 can grow and uptake NO3 rapidly at lower NO3 concentrations. Furthermore, growth rates and NO3 uptake of the species largely change when NO3 concentrations change near KGR-NO3 and KUT-NO3. The range of NO3 concentrations in the waters, collected from April 2015 to October 2018, from Taean (West Sea of Korea) and Yeosu (South Sea of Korea) from which A. affine and A. fraterculus were isolated, were 1.6–10.7 and 0.8–26.4 μM, respectively (Kang et al. 2019). Furthermore, the range of NO3 concentrations in Junk Bay, Hong Kong and Ria de Vigo, northwest Spain, from which A. affine was found were 0–15.0 and 0–30.0 μM, respectively (Fraga et al. 1989, Nogueira et al. 1997, Hodgkiss and Lu 2004, Lee et al. 2009). The KGR-NO3 of A. affine (8.7 μM) and A. fraterculus (5.4 μM) fall in the ranges of the NO3 concentrations in the waters off Taean and Yeosu, Korea, Junk Bay, Hong Kong, and Ria de Vigo, Spain. Therefore, a small change in NO3 concentrations in these waters may sometimes cause a large change in growth rates of A. affine and A. fraterculus.
The range of NO3 concentrations in the waters, collected from April 2015 to October 2018, from 9 stations located in South Sea of Korea in which A. affine and A. fraterculus have caused red tides or harmful algal blooms, was 0.4–102.9 μM (Kang et al. 2019). Moreover, the range of NO3 concentrations in Seto Inland Sea, Japan in which A. affine and A. fraterculus were found was 5.4–70.0 μM (Nakanishi et al. 1996, Montani et al. 1998, Nagai et al. 2009). The KUT-NO3 of A. affine (37.5 μM) and A. fraterculus (44.4 μM) fall in the ranges of the NO3 concentrations in South Sea of Korea and Seto Inland Sea. Therefore, a small change in NO3 concentrations in South Sea of Korea and Seto Inland Sea may sometimes cause a large change in the NO3 uptake of A. affine and A. fraterculus. In addition, the KUT-NO3 of A. affine was lower than that of A. fraterculus, while the KGR-NO3 of A. fraterculus was lower than that of A. affine. Therefore, A. affine rapidly uptakes NO3 at lower NO3 concentrations than A. fraterculus, but A. fraterculus rapidly grows at lower NO3 concentrations than A. affine.
Interestingly, the KGR-NO3 of A. affine and A. fraterculus were much lower than their KUT-NO3. That is, the growth rates of A. affine and A. fraterculus became saturated at the NO3 concentrations at which NO3 uptakes increased. For a cell to divide, the cell should uptake a certain amount of NO3. Thus, NO3 acquired by the cell may not have been high enough to be used for cell division. Similar patterns are often observed in feeding by mixotrophic and heterotrophic protists; growth rate of a mixotrophic or heterotrophic protist on algal prey become saturated at prey concentrations at which its ingestion rates increase (Jeong et al. 2007, 2014, 2018a, 2018b, Lim et al. 2014). In conclusion, the values of KGR-NO3 and KUT-NO3 of a species should be determined separately because they can be different from each other. The nutrient repletion method can be a useful tool for determining KGR-NO3 and KUT-NO3 of the species as a function of NO3 concentration simultaneously.
The maximum NO3 uptake of A. affine (ca. 13–16 pM cell−1 d−1) is comparable to that of Alexandrium minutum (4.3–16.8 pM cell−1 d−1) and Alexandrium tamiyavanichii (ca. 10–20 pM cell−1 d−1), but the maximum NO3 uptake of A. fraterculus (ca. 30 pM cell−1 d−1) is greater than that of these Alexandrium species (Lim et al. 2006, Maguer et al. 2007). The size of A. fraterculus (32.3 μm) is larger than that of A. minimum (20.5 μm), comparable to that of A. affine (31.4 μm), but smaller than that of A. tamiyavanichii (38.6 μm). The maximum NO3 uptake of these Alexandrium species was not significantly correlated with their size (p > 0.1, linear regression ANOVA). Thus, the maximum NO3 uptake of an Alexandrium species should be measured because it may not be calculated using the equation of a regression between the maximum NO3 uptake and size of Alexandrium species.
The maximum growth rate of A. fraterculus under continuous light conditions was much lower than that under light and dark conditions, whereas the maximum growth rate of A. affine grown under continuous light conditions was slightly higher than that under light and dark conditions. This evidence suggests that growth rate of A. fraterculus may be inhibited by continuous light. One scenario is that A. fraterculus has a strong circadian rhythm and a continuous light condition disturbs this rhythm. There have been many dinoflagellates species showing circadian rhythms (Prézelin et al. 1977, Knaust et al. 1998, Van Dolah et al. 2007, Dapena et al. 2015); A. minutum showed nuclear and morphological changes during cell cycle and growth, indicating a strong circadian rhythm (Dapena et al. 2015). In contrast, to our best knowledge, there has only been one dinoflagellate species identified as showing inhibition of growth under a continuous light condition; the growth rate of Tripos (Ceratium) ranipes under a continuous light of 320 μmol photons m−2 s−1 was much lower than that under light and dark cycle (Brand and Guillard 1981). Thus, the present study adds A. fraterculus to a few dinoflagellate species showing inhibition of growth under a continuous light condition. Another scenario is that an excessive amount of photons inhibits the operation of photosystems of A. fraterculus (Richardson et al. 1983, Krause 1988). The amount of photons produced by a continuous light is ca. 40% greater than that by 14-h illumination. Thus, the amount of photons produced by a continuous light at 100 μmol photons m−2 s−1 is equivalent to that at 140 μmol photons m−2 s−1 under 14-h light : 10-h dark cycle. The autotrophic growth of the mixotrophic dinoflagellate Takayama helix is known to be inhibited at 115 μmol photons m−2 s−1 under 14-h light : 10-h dark cycle (Ok et al. 2019). Therefore, it is worthwhile to explore possible inhibition of growth of A. fraterculus at high light intensities under 14-h light : 10-h dark cycle to test this hypothesis.
ACKNOWLEDGEMENTSWe thank An Suk Lim, Sung Yeon Lee, and Se Hyun Jang for technical support. This research was supported by the Useful Dinoflagellate program of Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) and the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2015M1A5A1041806; NRF-2017R1E1A1A01074419) award to HJJ and (NRF-2019R1C1C1008546) to KHL.
Fig. 1(A–C) Change in the concentration (abundance, AB) of Alexandrium affine (cells mL−1) as a function of elapsed time (d) under an illumination of 100 μmol photons m−2 s−1 on a 14-h light : 10-h dark (LD) cycle. The concentration of A. affine provided in the normal scale (A) and natural log (Ln) scale at Day 0 to 14 (B) and at Day 1 to 9 (C). (D–F) Change in the NO3 (D) and PO4 (E) concentrations and ratio of NO3 relative to PO4 (F) concentration as a function of elapsed time (d). Symbols represent each treatment. The curves in (B) and (C) are fitted to a linear regression using all the treatments obtained from Day 0 to 14 (B) and from Day 1 to 9 (C). (B) Ln (AB) = 0.332 (d) + 4.89, r2 = 0.957; (C) Ln (AB) = 0.425 (d) + 4.50, r2 = 0.993. 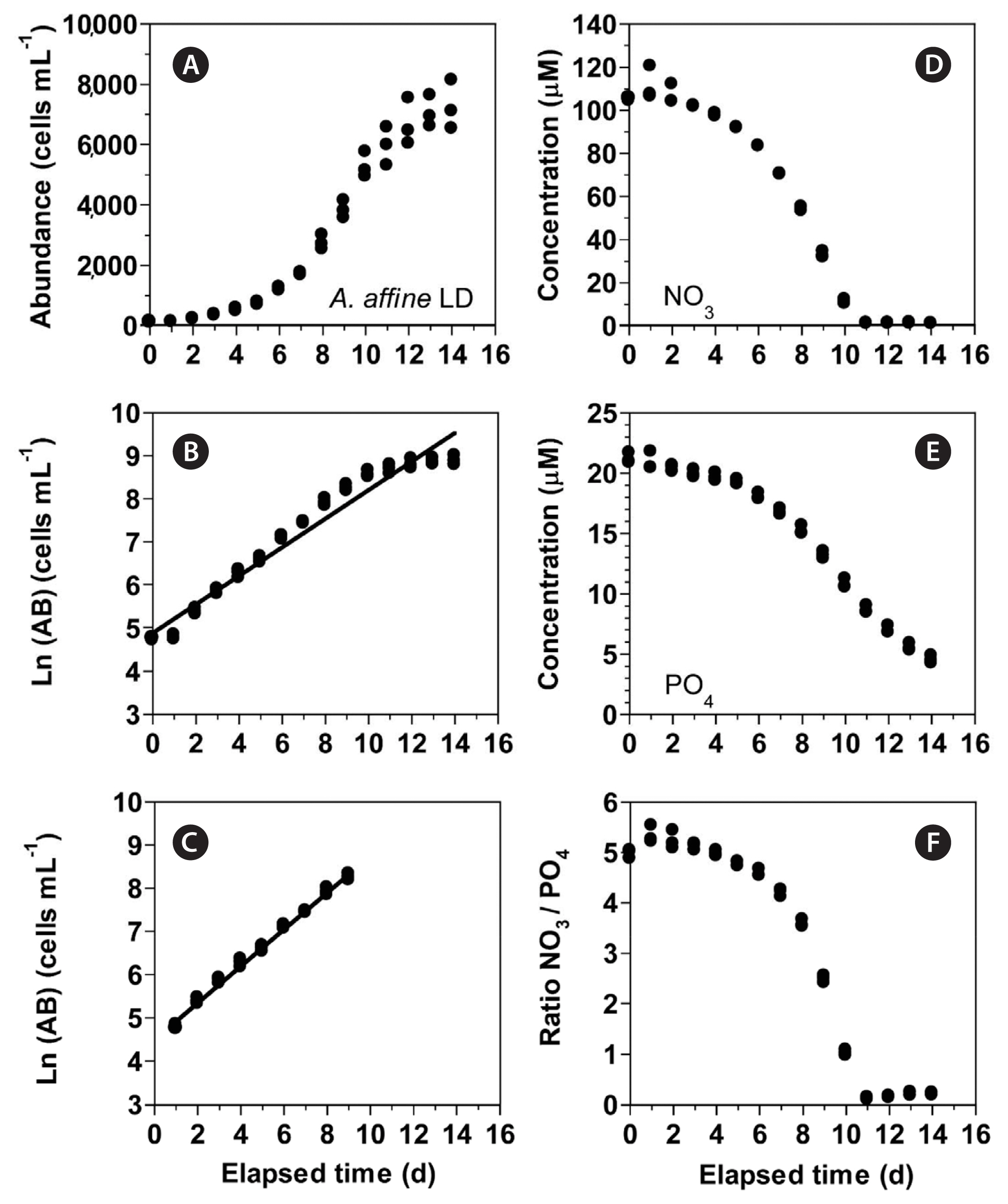
Fig. 2Growth rates (GR, d−1) of Alexandrium affine as a function of NO3 (A) and PO4 (B) concentration under an illumination of 100 μmol photons m−2 s−1 on a 14-h light : 10-h dark (LD) cycle. Symbols represent each treatment. The curve in (A) is fitted to the Michaelis-Menten equation using all the treatments obtained from Day 4 to 14 and that in (B) to a linear regression using all the treatment obtained from Day 4 to 14. (A) GR = 0.447 [NO3/(8.67 + NO3)], r2 = 0.733; (B) GR = 0.0278 (PO4) − 0.0934, r2 = 0.676. 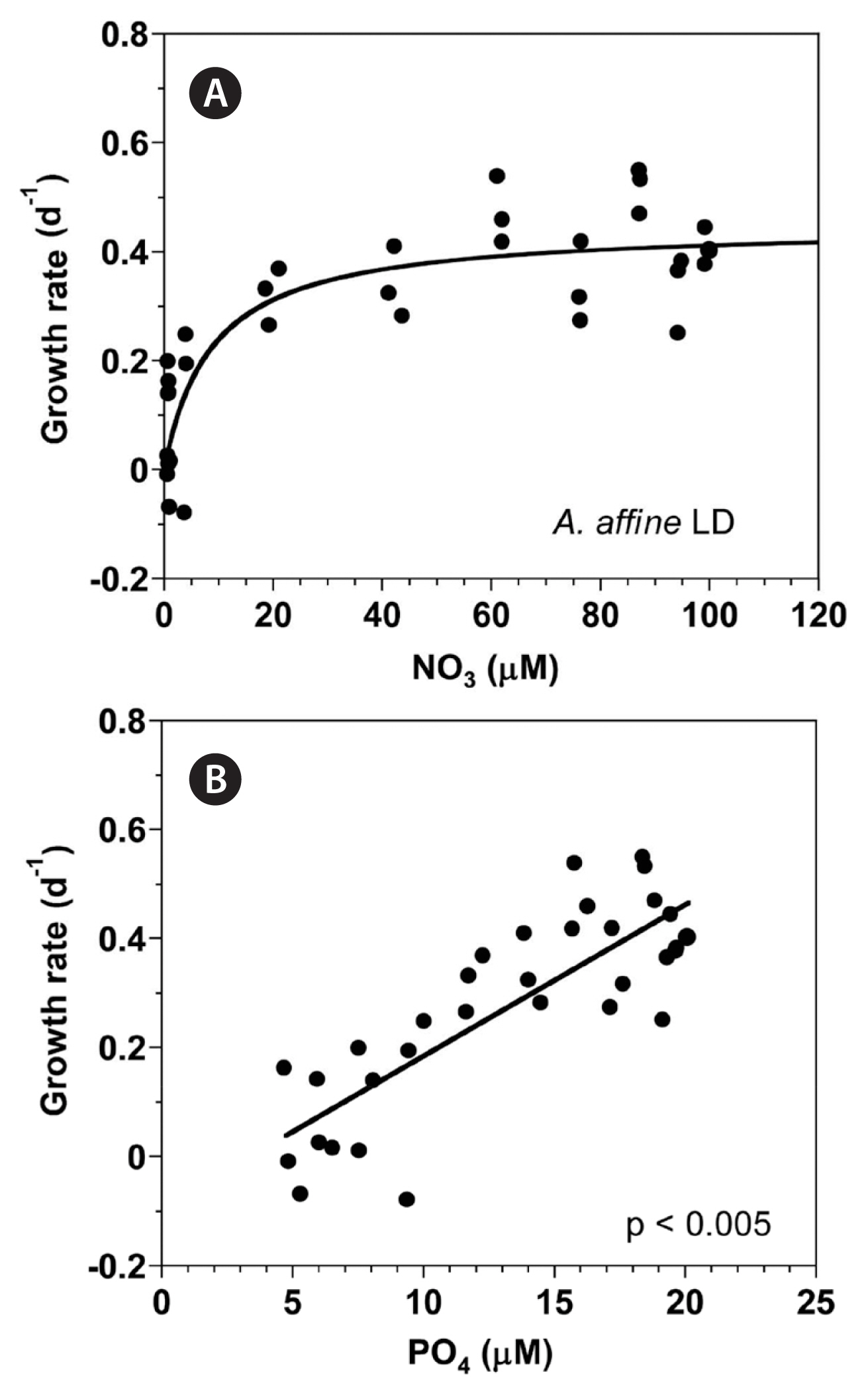
Fig. 3(A) The NO3 uptake (NUT, pM cell−1 d−1) of Alexandrium affine as a function of NO3 concentrations (μM) under an illumination of 100 μmol photons m−2 s−1 on a 14-h light : 10-h dark (LD) cycle. (B) The PO4 uptake (PUT, pM cell−1 d−1) of A. affine as a function of PO4 concentrations (μM). Symbols represent each treatment. The curve in (A) is fitted to the Michaelis-Menten equation using all the treatments obtained from Day 4 to 14 and that in (B) to a linear regression using all the treatment obtained from Day 4 to 14. (A) NUT = 12.9 [NO3/(37.5 + NO3)], r2 = 0.971; (B) PUT = 0.05 (PO4) − 0.0887, r2 = 0.676. 
Fig. 4(A–C) Change in the concentration of Alexandrium fraterculus (cells mL−1) as a function of elapsed time (d) under an illumination of 100 μmol photons m−2 s−1 on a 14-h light : 10-h dark (LD) cycle. The concentration of A. fraterculus provided in the normal scale (A) and natural log (Ln) scale at Day 0 to 14 (B) and at Day 1 to 9 (C). (D–F) Change in the NO3 (D) and PO4 (E) concentrations and ratio of NO3 relative to PO4 (F) concentration as a function of elapsed time (d). Symbols represent each treatment. The curves in (B) and (C) are fitted to a linear regression using all the treatments obtained from Day 0 to 14 (B) and from Day 1 to 9 (C). (B) Ln (AB) = 0.298 (d) + 4.87, r2 = 0.937; (C) Ln (AB) = 0.389 (d) + 4.52, r2 = 0.986. 
Fig. 5Growth rates (GR, d−1) of Alexandrium fraterculus as a function of NO3 (A) and PO4 (B) concentration under an illumination of 100 μmol photons m−2 s−1 on a 14-h light : 10-h dark (LD) cycle. Symbols represent each treatment. The curve in (A) is fitted to the Michaelis-Menten equation using all the treatments obtained from Day 0 to 14 and that in (B) to a linear regression using all the treatment obtained from Day 0 to 14. (A) GR = 0.422 [NO3/(5.38 + NO3)], r2 = 0.741; (B) GR = 0.0412 (PO4) − 0.41, r2 = 0.615. 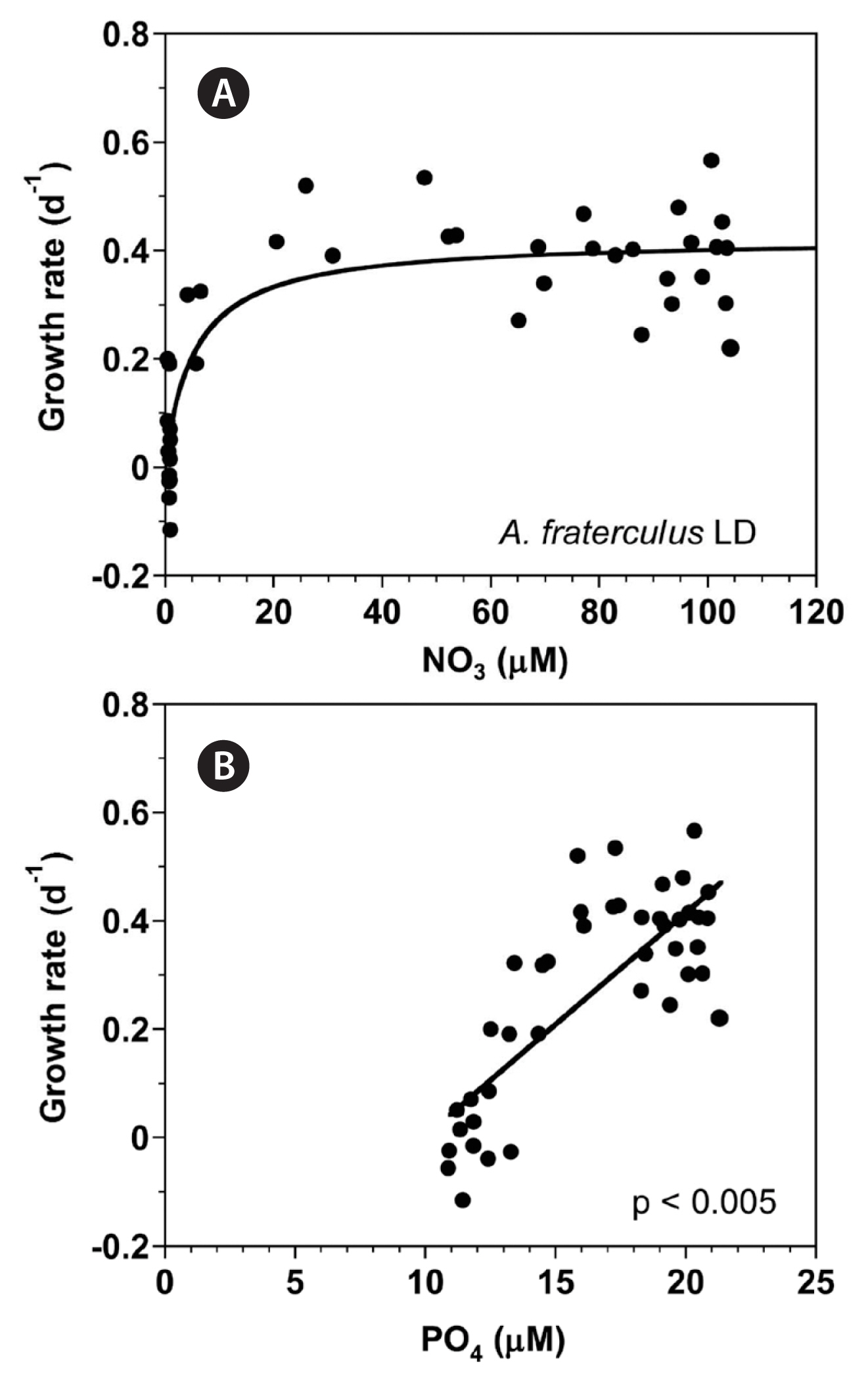
Fig. 6(A) The NO3 uptake (NUT, pM cell−1 d−1) of Alexandrium fraterculus as a function of NO3 concentrations (μM) under an illumination of 100 μmol photons m−2 s−1 on a 14-h light : 10-h dark (LD) cycle. (B) The PO4 uptake (PUT, pM cell−1 d−1) of A. fraterculus as a function of PO4 concentrations (μM). Symbols represent each treatment. The curve in (A) is fitted to the Michaelis-Menten equation using all the treatments obtained from Day 2 to 14 and that in (B) to a linear regression using all the treatment obtained from Day 2 to 14. (A) NUT = 30.1 [NO3/(44.4 + NO3)], r2 = 0.843; (B) PUT = 0.164 (PO4) − 1.86, r2 = 0.548. 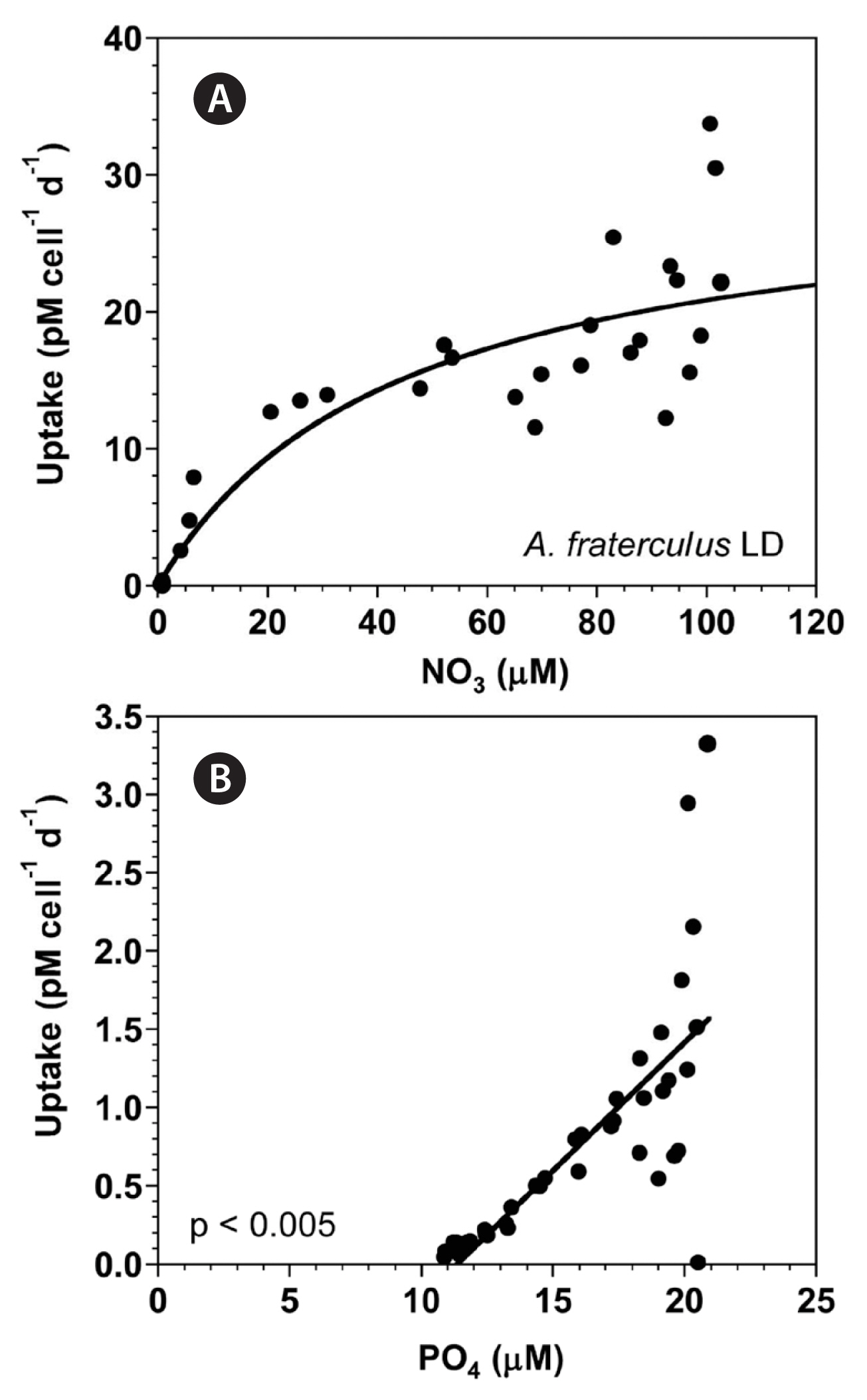
Fig. 7(A–C) Change in the concentration of Alexandrium affine (cells mL−1) as a function of elapsed time (d) at 100 μmol photons m−2 s−1 under continuous light conditions (CL). The concentration of A. affine provided in the normal scale (A) and natural log (Ln) scale at Day 0 to 14 (B) and at Day 1 to 9 (C). (D–F) Change in the NO3 (D) and PO4 (E) concentrations and ratio of NO3 relative to PO4 (F) concentration as a function of elapsed time (d). Symbols represent each treatment. The curves in (B) and (C) are fitted to a linear regression using all the treatments obtained from Day 0 to 14 (B) and from Day 1 to 9 (C). (B) Ln (AB) = 0.324 (d) + 4.79, r2 = 0.961; (C) Ln (AB) = 0.405 (d) + 4.46, r2 = 0.986. 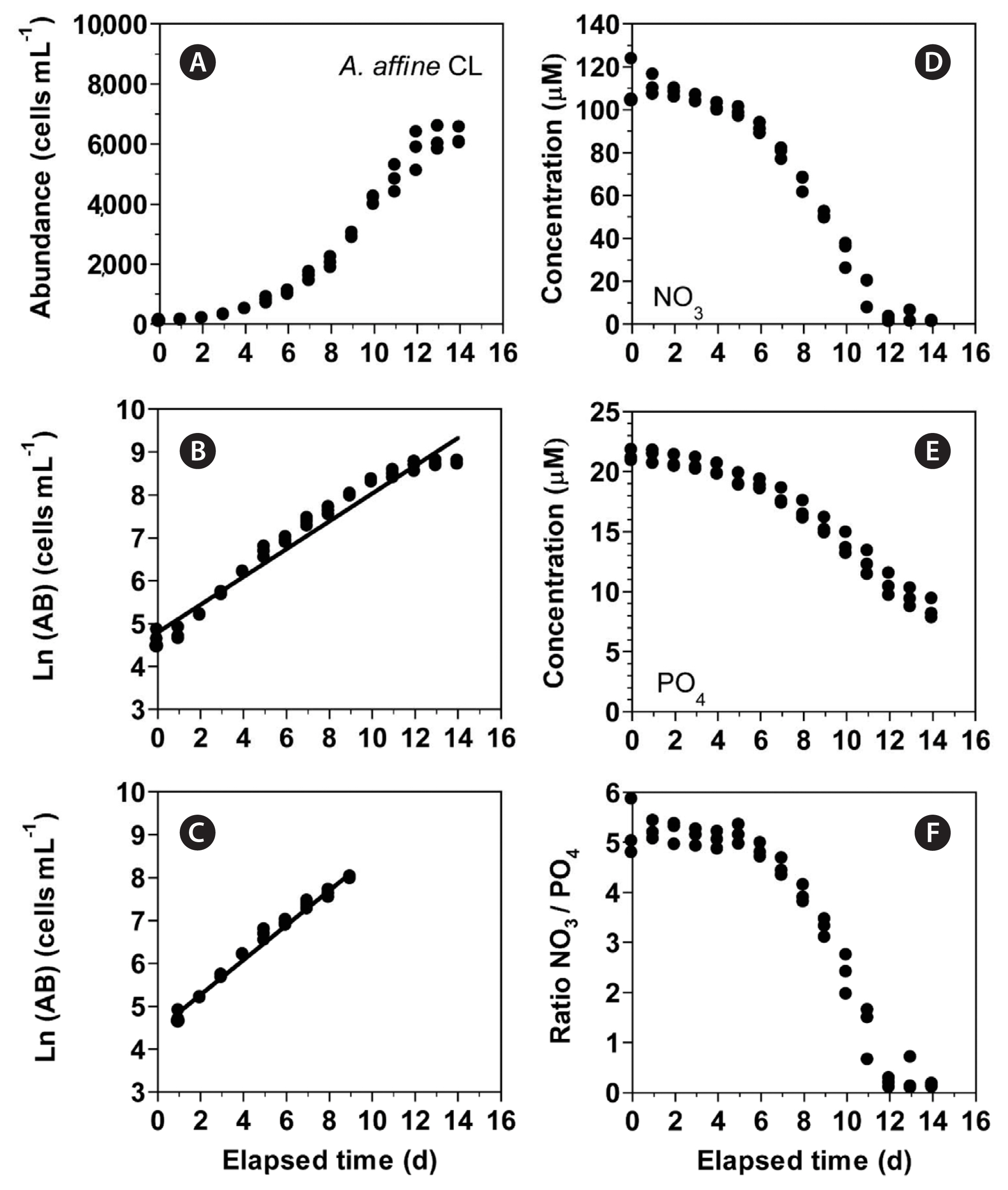
Fig. 8Growth rates (GR, d−1) of Alexandrium affine as a function of NO3 (A) and PO4 (B) concentration at 100 μmol photons m−2 s−1 under continuous light conditions (CL). Symbols represent each treatment. The curve in (A) is fitted to the Michaelis-Menten equation using all the treatments obtained from Day 0 to 14 and that in (B) to a linear regression using all the treatment obtained from Day 0 to 14. (A) GR = 0.458 [NO3/(21.5 + NO3)], r2 = 0.439; (B) GR = 0.0281 (PO4) − 0.171, r2 = 0.417. 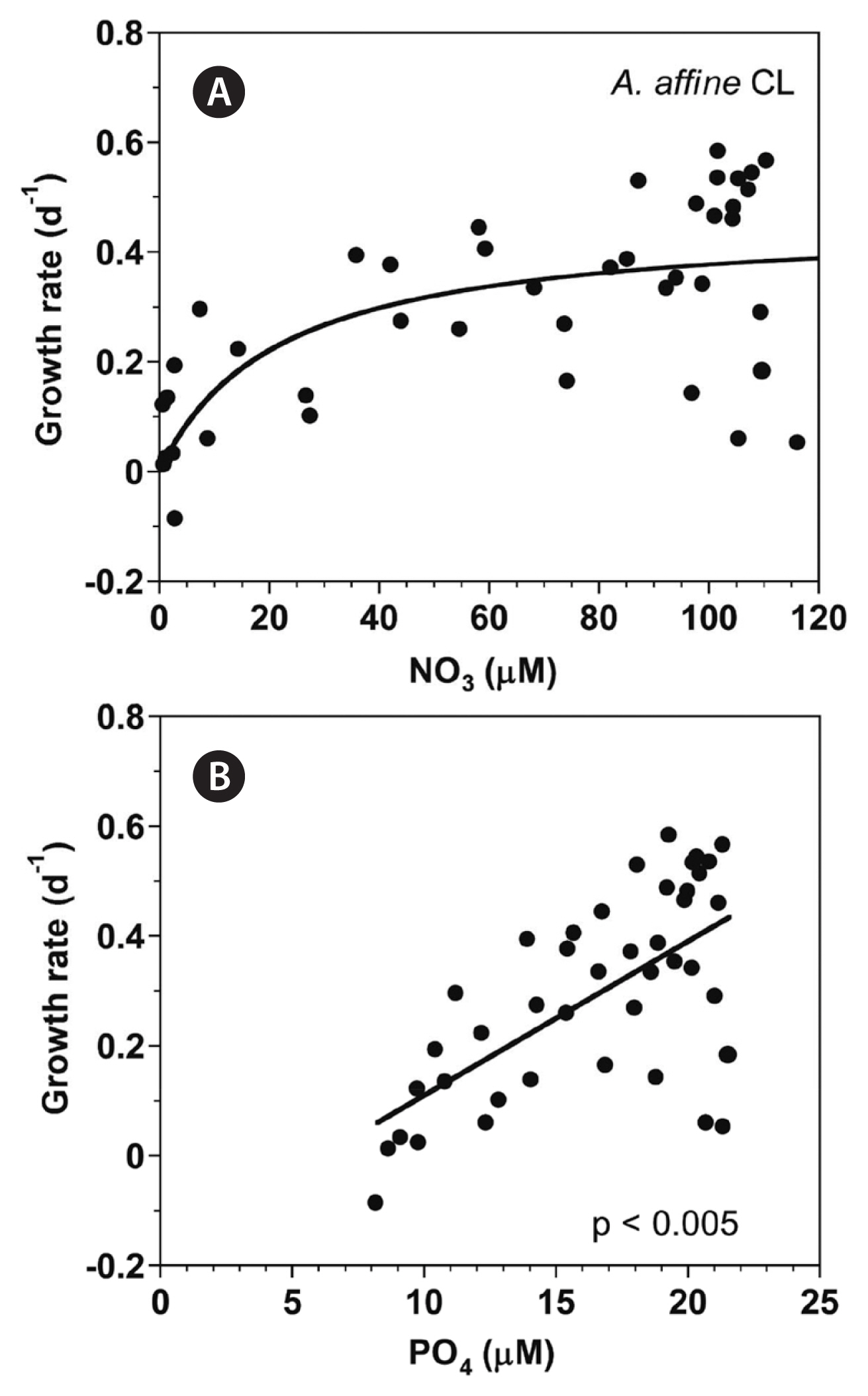
Fig. 9(A) The NO3 uptake (NUT, pM cell−1 d−1) of Alexandrium affine as a function of NO3 concentrations (μM) at 100 μmol photons m−2 s−1 under continuous light conditions (CL). (B) The PO4 uptake (PUT, pM cell−1 d−1) of A. affine as a function of PO4 concentrations (μM). Symbols represent each treatment. The curve in (A) is fitted to the Michaelis-Menten equation using all the treatments obtained from Day 3 to 14 and that in (B) to a linear regression using all the treatment obtained from Day 3 to 14. (A) NUT = 16.4 [NO3/(83.3 + NO3)], r2 = 0.606; (B) PUT = 0.0764 (PO4) − 0.592, r2 = 0.603. 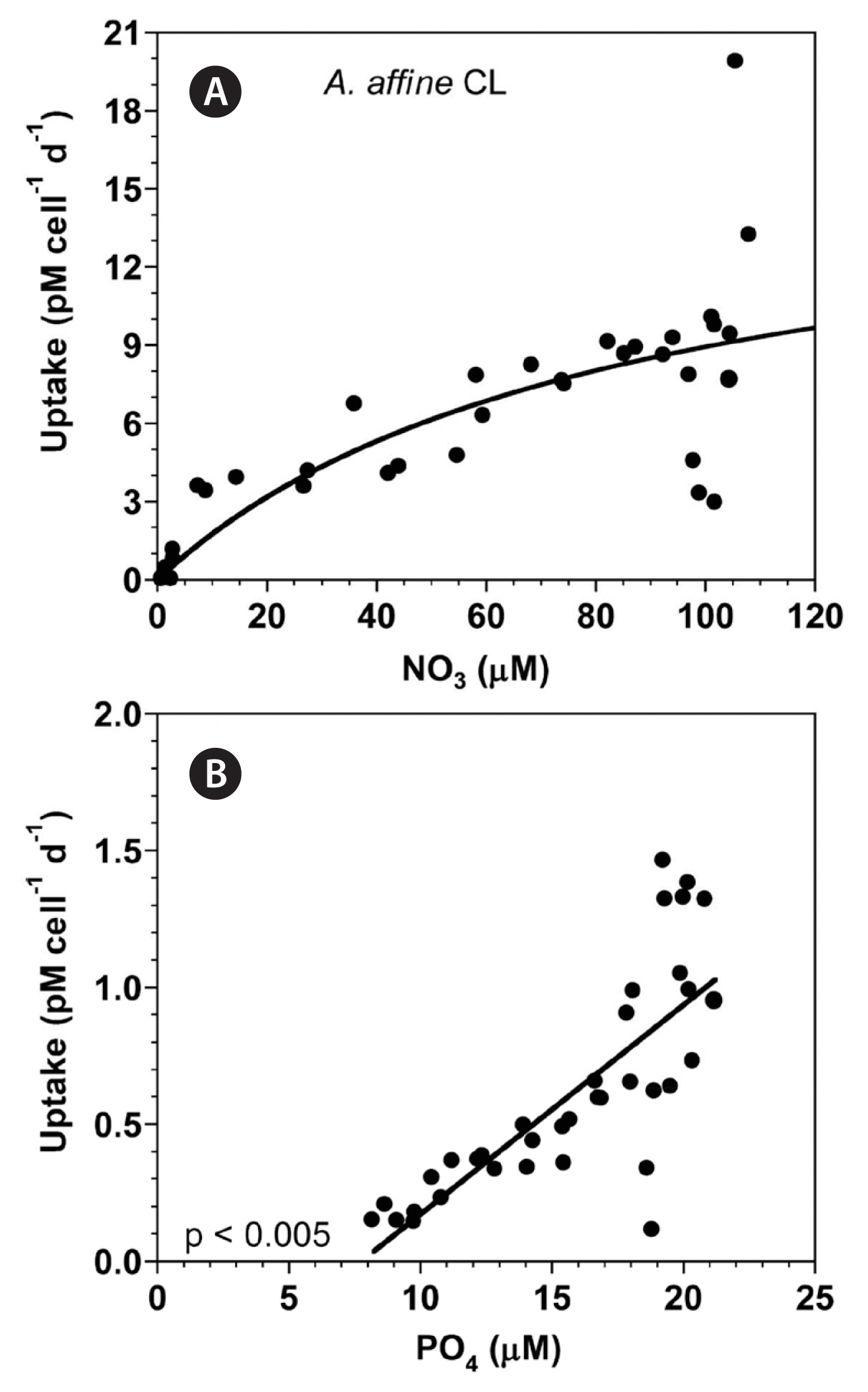
Fig. 10(A–C) Change in the concentration of Alexandrium fraterculus (cells mL−1) as a function of elapsed time (d) at 100 μmol photons m−2 s−1 under continuous light conditions (CL). The concentration of A. fraterculus provided in the normal scale (A) and natural log (Ln) scale at Day 0 to 14 (B) and at Day 2 to 9 (C). (D–F) Change in the NO3 (D) and PO4 (E) concentrations and ratio of NO3 relative to PO4 (F) concentration as a function of elapsed time (d). Symbols represent each treatment. The curves in (B) and (C) are fitted to a linear regression using all the treatments obtained from Day 0 to 14 (B) and from Day 2 to 9 (C). (B) Ln (AB) = 0.217 (d) + 4.75, r2 = 0.936; (C) Ln (AB) = 0.295 (d) + 4.43, r2 = 0.951. 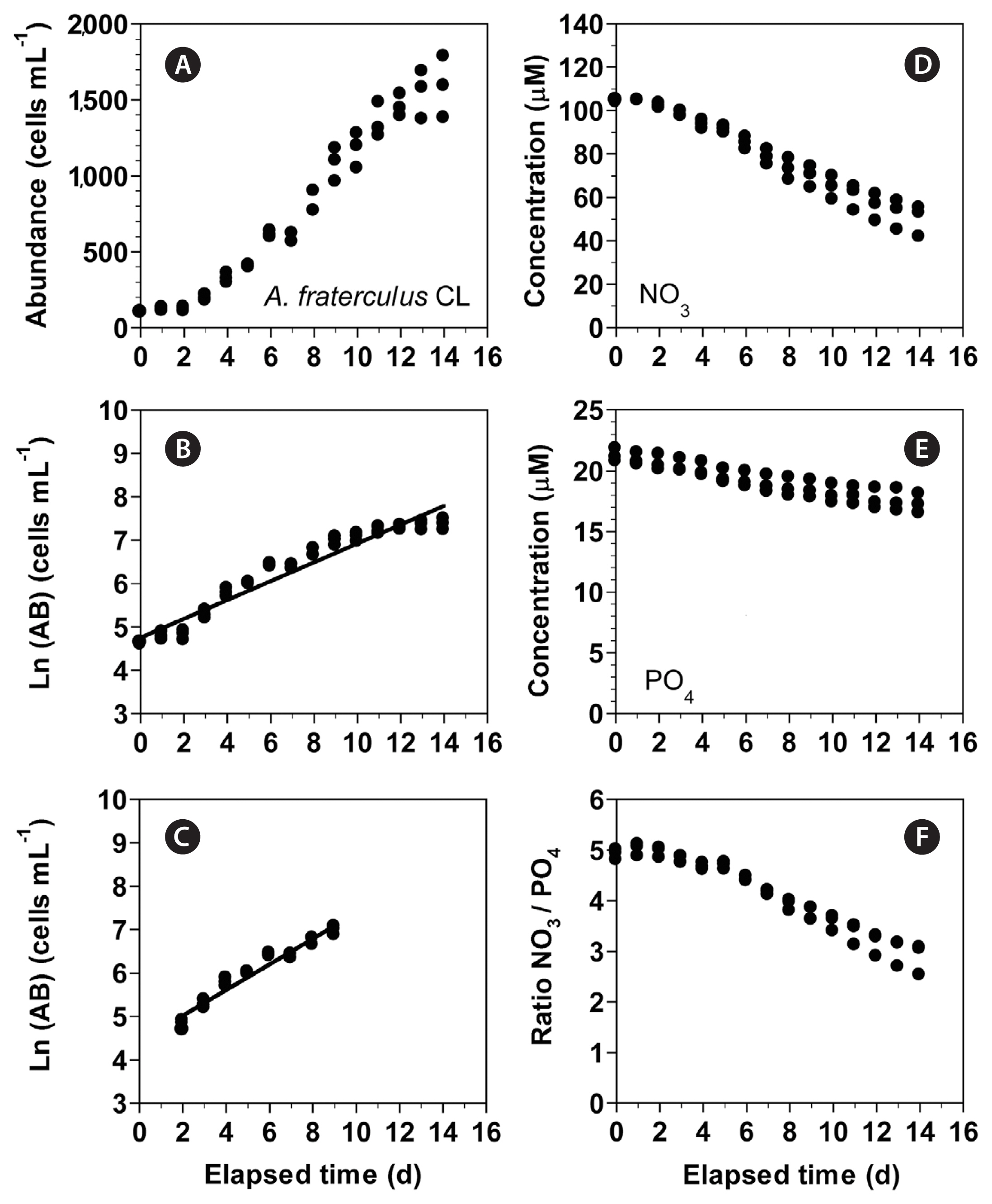
Fig. 11Growth rates (GR, d−1) of Alexandrium fraterculus as a function of NO3 (A) and PO4 (B) concentration at 100 μmol photons m−2 s−1 under continuous light conditions (CL). Symbols represent each treatment. The curve in (A) is fitted to a linear regression using all the treatments obtained from Day 2 to 14 and that in (B) from Day 2 to 14. (A) GR = 0.00508 (NO3) − 0.193, r2 = 0.224; (B) GR = 0.0623 (PO4) − 0.98, r2 = 0.165. 
Fig. 12(A) The NO3 uptake (NUT, pM cell−1 d−1) of Alexandrium fraterculus as a function of NO3 concentrations (μM) at 100 μmol photons m−2 s−1 under continuous light conditions (CL). (B) The PO4 uptake (PUT, pM cell−1 d−1) of A. fraterculus as a function of PO4 concentrations (μM). Symbols represent each treatment. The curve in (A) is fitted to a linear regression using all the treatments obtained from Day 2 to 14 and that in (B) from Day 2 to 14. (A) NUT = 0.362 (NO3) − 18.7, r2 = 0.656; (B) PUT = 0.447 (PO4) − 7.74, r2 = 0.502. 
Table 1Conditions for the isolation of Alexandrium affine and A. fraterculus used in this study
Table 2Experimental design and the light and nutrient conditions Table 3Maximum growth rates (μmax, d−1) of Alexandrium affine and A. fraterculus, half saturation constant for growth rate as a function of nitrate concentration (KGR-NO3, μM), nitrate maximum uptake (Vmax-NO3, pM cell−1 d−1), half saturation constant for the nitrate uptake as a function of nitrate concentration (KUT-NO3, μM) and conditions for growth
ESD, equivalent spherical diameter (μm); T, temperature (°C); S, salinity; L, light intensity (μmol photons m−2 s−1); L : D cycle, light and dark cycle (hour : hour); NO3, range of concentration of nitrate used in experiment (μM); CL, continuous light. a Data from Kang et al. (2018). REFERENCESAnderson, DM., Alpermann, TJ., Cembella, AD., Collos, Y., Masseret, E. & Montresor, M. 2012. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 14:10–35.
Band-Schmidt, CJ., Lechuga-Devéze, CH., Kulis, DM. & Anderson, DM. 2003. Culture studies of Alexandrium affine (Dinophyceae), a non-toxic cyst forming dinoflagellate from Bahía Concepción, Gulf of California. Bot Mar. 46:44–54.
Basti, L., Nagai, S., Go, J., Okano, S., Nagai, K., Watanabe, R., Suzuki, T. & Tanaka, Y. 2015. Differential inimical effects of Alexandrium spp. and Karenia spp. on cleavage, hatching, and two larval stages of Japanese pearl oyster Pinctada fucata martensii
. Harmful Algae. 43:1–12.
Blossom, HE., Daugbjerg, N. & Hansen, PJ. 2012. Toxic mucus traps: a novel mechanism that mediates prey uptake in the mixotrophic dinoflagellate Alexandrium pseudogonyaulax
. Harmful Algae. 17:40–53.
Brand, LE. & Guillard, RRL. 1981. The effects of continuous light and light intensity on the reproduction rates of twenty-two species of marine phytoplankton. J Exp Mar Biol Ecol. 50:119–132.
Dapena, C., Bravo, I., Cuadrado, A. & Figueroa, RI. 2015. Nuclear and cell morphological changes during the cell cycle and growth of the toxic dinoflagellate Alexandrium minutum
. Protist. 166:146–160.
Dias, J., Muñoz, J., Huisman, J. & McDonald, J. 2015. Biosecurity monitoring of Harmful Algal Bloom (HAB) species in Western Australian waters: first confirmed record of Alexandrium catenella (Dinophyceae). Bioinvasions Rec. 4:233–241.
Eckford-Soper, LK., Bresnan, E., Lacaze, J-P., Green, DH. & Davidson, K. 2016. The competitive dynamics of toxic Alexandrium fundyense and non-toxic Alexandrium tamarense: the role of temperature. Harmful Algae. 53:135–144.
Fraga, S., Gallager, SM. & Anderson, DM. 1989. Chain-forming dinoflagellates: an adaptation to red tides. In : Okaichi T, Anderson DM, Nemoto T, editors Red Tides: Biology, Environmental Science and Toxicology. Elsevier, New York, 281–284.
Guillard, RRL. & Hargraves, PE. 1993.
Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia. 32:234–236.
Guillard, RRL. & Ryther, JH. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol. 8:229–239.
Hansen, G., Daugbjerg, N. & Franco, JM. 2003. Morphology, toxin composition and LSU rDNA phylogeny of Alexandrium minutum (Dinophyceae) from Denmark, with some morphological observations on other European strains. Harmful Algae. 2:317–335.
Hatfield, RG., Bean, T., Turner, AD., Lees, DN., Lowther, J., Lewis, A. & Baker-Austin, C. 2019. Development of a TaqMan qPCR assay for detection of Alexandrium spp. and application to harmful algal bloom monitoring. Toxicon X. 2:100011 pp.
Hodgkiss, IJ. & Lu, S. 2004. The effects of nutrients and their ratios on phytoplankton abundance in Junk Bay, Hong Kong. In : Ang PO, editor Asian Pacific Phycology in the 21st Century: Prospects and Challenges. Springer, Dordrecht, 215–229.
Jacobson, DM. & Anderson, DM. 1986. Thecate heterotrophic dinoflagellates: feeding behavior and mechanisms. J Phycol. 22:249–258.
Jauzein, C., Labry, C., Youenou, A., Quéré, J., Delmas, D. & Collos, Y. 2010. Growth and phosphorus uptake by the toxic dinoflagellate Alexandrium catenella (Dinophyceae) in response to phosphate limitation. J Phycol. 46:926–936.
Jeong, HJ., Kang, HC., You, JH. & Jang, SH. 2018a. Interactions between the newly described small- and fast-swimming mixotrophic dinoflagellate Yihiella yeosuensis and common heterotrophic protists. J Eukaryot Microbiol. 65:612–626.
Jeong, HJ., Kim, JS., Song, JY., Kim, JH., Kim, TH., Kim, SK. & Kang, NS. 2007. Feeding by protists and copepods on the heterotrophic dinoflagellates Pfiesteria piscicida, Stoeckeria algicida, and Luciella masanensis
. Mar Ecol Prog Ser. 349:199–211.
Jeong, HJ., Lim, AS., Franks, PJS., Lee, KH., Kim, JH., Kang, NS., Lee, MJ., Jang, SH., Lee, SY., Yoon, EY., Park, JY., Yoo, YD., Seong, KA., Kwon, JE. & Jang, TY. 2015. A hierarchy of conceptual models of red-tide generation: nutrition, behavior, and biological interactions. Harmful Algae. 47:97–115.
Jeong, HJ., Lim, AS., Lee, K., Lee, MJ., Seong, KA., Kang, NS., Jang, SH., Lee, KH., Lee, SY., Kim, MO., Kim, JH., Kwon, JE., Kang, HC., Kim, JS., Yih, W., Shin, K., Jang, PK., Ryu, J-H., Kim, SY., Park, JY. & Kim, KY. 2017. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: I. temporal variations in three-dimensional distributions of red-tide organisms and environmental factors. Algae. 32:101–130.
Jeong, HJ., Lim, AS., Yoo, YD., Lee, MJ., Lee, KH., Jang, TY. & Lee, K. 2014. Feeding by heterotrophic dinoflagellates and ciliates on the free-living dinoflagellate Symbiodinium sp. (Clade E). J Eukaryot Microbiol. 61:27–41.
Jeong, HJ., Park, JY., Nho, JH., Park, MO., Ha, JH., Seong, KA., Jeng, C., Seong, CN., Lee, KY. & Yih, WH. 2005a. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus
. Aquat Microb Ecol. 41:131–143.
Jeong, HJ., Yoo, YD., Kim, JS., Seong, KA., Kang, NS. & Kim, TH. 2010. Growth, feeding, and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J. 45:65–91.
Jeong, HJ., Yoo, YD., Park, JY., Song, JY., Kim, ST., Lee, SH., Kim, KY. & Yih, WH. 2005b. Feeding by the phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. Aquat Microb Ecol. 40:133–150.
Jeong, HJ., You, JH., Lee, KH., Kim, SJ. & Lee, SY. 2018b. Feeding by common heterotrophic protists on the mixotrophic alga Gymnodinium smaydae (Dinophyceae), one of the fastest growing dinoflagellates. J Phycol. 54:734–743.
Kang, HC., Jeong, HJ., Kim, SJ., You, JH. & Ok, JH. 2018. Differential feeding by common heterotrophic protists on 12 different Alexandrium species. Harmful Algae. 78:106–117.
Kang, HC., Jeong, HJ., Ok, JH., You, JH., Jang, SH., Lee, SY., Lee, KH., Park, JY. & Rho, J-R. 2019. Spatial and seasonal distributions of the phototrophic dinoflagellate Biecheleriopsis adriatica (Suessiaceae) in Korea: quantification using qPCR. Algae. 34:111–126.
Katsuo, D., Kim, D., Yamaguchi, K., Matsuyama, Y. & Oda, T. 2007. A new simple screening method for the detection of cytotoxic substances produced by harmful red tide phytoplankton. Harmful Algae. 6:790–798.
Kim, JH. 2017. Spatiotemporal distribution and a survival strategy of harmful dinoflagellates Alexanderium spp. in Korean coastal waters and application to controlling scuticociliates. MS thesis. Seoul National University, Seoul, Korea, 136 pp.
Knaust, R., Urbig, T., Li, L., Taylor, W. & Hastings, JW. 1998. The circadian rhythm of bioluminescence in Pyrocystis is not due to differences in the amount of luciferase: a comparative study of three bioluminescent marine dinoflagellates. J Phycol. 34:167–172.
Krause, GH. 1988. Photoinhibition of photosynthesis: an evaluation of damaging and protective mechanisms. Physiol Plant. 74:566–574.
Lagos, N. 2003. Paralytic shellfish poisoning phycotoxins: occurrence in South America. Comments Toxicol. 9:175–193.
Leaw, CP., Lim, PT., Ng, BK., Cheah, MY., Ahmad, A. & Usup, G. 2005. Phylogenetic analysis of Alexandrium species and Pyrodinium bahamense (Dinophyceae) based on theca morphology and nuclear ribosomal gene sequence. Phycologia. 44:550–565.
Lee, FW., Morse, D. & Lo, SC-L. 2009. Identification of two plastid proteins in the dinoflagellate Alexandrium affine that are substantially down-regulated by nitrogen-depletion. J Proteome Res. 8:5080–5092.
Lee, J., Kim, DY. & Lee, J. 1998. Community dynamics and distribution of dinoflagellates and their cysts in Masan-Chinhae Bay, Korea. Fish Aquat Sci. 1:283–292.
Lee, KH., Jeong, HJ., Kim, HJ. & Lim, AS. 2017. Nitrate uptake of the red tide dinoflagellate Prorocentrum micans measured using a nutrient repletion method: effect of light intensity. Algae. 32:139–153.
Lee, KH., Jeong, HJ., Kwon, JE., Kang, HC., Kim, JH., Jang, SH., Park, JY., Yoon, EY. & Kim, JS. 2016. Mixotrophic ability of the phototrophic dinoflagellates Alexandrium andersonii, A. affine, and A. fraterculus
. Harmful Algae. 59:67–81.
Lee, KH., Jeong, HJ., Lee, K., Franks, PJS., Seong, KA., Lee, SY., Lee, MJ., Jang, SH., Potvin, E., Lim, AS., Yoon, EY., Yoo, YD., Kang, NS. & Kim, KY. 2019. Effects of warming and eutrophication on coastal phytoplankton production. Harmful Algae. 81:106–118.
Li, T., Yu, R. & Zhou, M-J. 2009. Demand and adsorption strategies of phosphorus of Alexandrium catenella isolated from East China Sea. Mar Environ Sci. 28:355–359.
Lim, AS., Jeong, HJ., Jang, TY., Yoo, YD., Kang, NS., Yoon, EY. & Kim, GH. 2014. Feeding by the newly described heterotrophic dinoflagellate Stoeckeria changwonensis: a comparison with other species in the family Pfiesteriaceae. Harmful Algae. 36:11–21.
Lim, AS., Jeong, HJ., Kim, JH., Jang, SH., Lee, MJ. & Lee, K. 2015. Mixotrophy in the newly described dinoflagellate Alexandrium pohangense: a specialist for feeding on the fast-swimming ichthyotoxic dinoflagellate Cochlodinium polykrikoides
. Harmful Algae. 49:10–18.
Lim, P-T., Leaw, C-P., Kaga, S., Sekiguchi, K. & Ogata, T. 2007. Growth responses of five non toxic Alexandrium species (dinophyceae) to temperature and salinity. Mar. Res. Indonesia. 32:189–195.
Lim, P., Leaw, C., Usup, G., Kobiyama, A., Koike, K. & Ogata, T. 2006. Effects of light and temperature on growth, nitrate uptake, and toxin production of two tropical dinofagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae). J Phycol. 42:786–799.
Lim, YK., Baek, SH., Lee, M., Kim, YO., Choi, K. & Kim, JH. 2019. Phytoplankton composition associated with physical and chemical variables during summer in the southern sea of Korea: implication of the succession of the two toxic dinoflagellates Cochlodinium (a.k.a. Margalefidinium) polykrikoides and Alexandrium affine
. J Exp Mar Biol Ecol. 516:51–66.
MacKenzie, L., de Salas, M., Adamson, J. & Beuzenberg, V. 2004. The dinoflagellate genus Alexandrium (Halim) in New Zealand coastal waters: comparative morphology, toxicity and molecular genetics. Harmful Algae. 3:71–92.
Maguer, J., L’Helguen, S., Madec, C., Labry, C. & Le Corre, P. 2007. Nitrogen uptake and assimilation kinetics in Alexandrium minutum (Dynophyceae): effect of N-limited growth rate on nitrate and ammonium interactions. J Phycol. 43:295–303.
McCarthy, PM. 2013. Census of Australian Marine Dinoflagellates, Available from: http://www.anbg.gov.au/abrs/Dinoflagellates/index_Dino.html
. Accessed Apr 5, 2019
Moita, MT. & Vilarinho, MG. 1999. Checklist of phytoplankton species off Portugal: 70 years 1929–1998 of studies. Port Acta Biol Sér B Sist. 18:5–50.
Montani, S., Magni, P., Shimamoto, M., Abe, N. & Okutani, K. 1998. The effect of a tidal cycle on the dynamics of nutrients in a tidal estuary in the Seto Inland Sea, Japan. J Oceanogr. 54:65–76.
Nagai, S., Nishitani, G., Takano, Y., Yoshida, M. & Takayama, H. 2009. Encystment and excystment under laboratory conditions of the nontoxic dinoflagellate Alexandrium fraterculus (Dinophyceae) isolated from the Seto Inland Sea, Japan. Phycologia. 48:177–185.
Nakanishi, K., Masao, A., Sako, Y., Ishida, Y., Muguruma, H. & Karube, I. 1996. Detection of the red tide-causing plankton Alexandrium affine by a piezoelectric immunosensor using a novel method of immobilizing antibodies. Anal Lett. 29:1247–1258.
Nguyen-Ngoc, L. 2004. An autecological study of the potentially toxic dinoflagellate Alexandrium affine isolated from Vietnamese waters. Harmful Algae. 3:117–129.
Nogueira, E., Pérez, FF. & Ríos, AF. 1997. Seasonal patterns and long-term trends in an estuarine upwelling ecosystem (Ría de Vigo, NW Spain). Estuar Coast Shelf Sci. 44:285–300.
Ok, JH., Jeong, HJ., Lim, AS., You, JH., Kang, HC., Kim, SJ. & Lee, SY. 2019. Effects of light and temperature on the growth of Takayama helix (Dinophyceae): mixotrophy as a survival strategy against photoinhibition. J Phycol. Advanced online publication. https://doi.org/10.1111/jpy.12907
Omachi, CY., Tamanaha, MDS. & Proença, LADO. 2007. Bloom of Alexandrium fraterculus in coastal waters off Itajaí, SC, Southern Brazil. Braz J Oceanogr. 55:57–61.
Park, J., Jeong, HJ., Yoo, YD. & Yoon, EY. 2013. Mixotrophic dinoflagellate red tides in Korean waters: distribution and ecophysiology. Harmful Algae. 30(Suppl 1):S28–S40.
Prézelin, BB., Meeson, BW. & Sweeney, BM. 1977. Characterization of photosynthetic rhythms in marine dinoflagellates: I. Pigmentation, photosynthetic capacity and respiration. Plant Physiol. 60:384–387.
Richardson, K., Beardall, J. & Raven, JA. 1983. Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol. 93:157–191.
Seong, KA., Jeong, HJ., Kim, S., Kim, GH. & Kang, JH. 2006. Bacterivory by co-occurring red-tide algae, heterotrophic nanoflagellates, and ciliates. Mar Ecol Prog Ser. 322:85–97.
Van Dolah, FM., Lidie, KB., Morey, JS., Brunelle, SA., Ryan, JC., Monroe, EA. & Haynes, BL. 2007. Microarray analysis of diurnal-and circadian-regulated genes in the florida red-tide dinoflagellate Karenia brevis (Dinophyceae) 1. J Phycol. 43:741–752.
Van Haren, H. & Compton, TJ. 2013. Diel vertical migration in deep sea plankton is finely tuned to latitudinal and seasonal day length. PLoS One. 8:e64435 pp.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||