ABSTRACTThe phototrophic dinoflagellate genus Scrippsiella is known to have a worldwide distribution. Here, we report for the first time, the occurrence of Scrippsiella lachrymosa in Korean waters. Unlike the other stains of S. lachrymosa whose cultures had been established from cysts in the sediments, the clonal culture of the Korean strain of S. lachrymosa was established from motile cells. When the sulcal plates of S. lachrymosa, which have not been fully described to date, were carefully examined using scanning electron microscopy, the Korean strain of S. lachrymosa clearly exhibited the anterior sulcal plate (s.a.), right sulcal plate (s.d.), left sulcal plate (s.s.), median sulcal plate (s.m.), and posterior sulcal plate (s.p.). When properly aligned, the large subunit (LSU) rDNA sequence of the Korean strain of S. lachrymosa was ca. 1% different from those of two Norwegian strains of S. lachrymosa, the only strains for which LSU sequences have been reported. The internal transcribed spacer (ITS) rDNA sequence of the Korean strain of S. lachrymosa was also ca. 1% different from those of the Scottish and Chinese strains and 3% different from those of the Canadian, German, Greek, and Portuguese strains. Thus, the Korean S. lachrymosa strain has unique LSU and ITS sequences. The abundances of S. lachrymosa in the waters of 28 stations, located in the East, West, and South Sea of Korea, were quantified in four seasons from January 2016 to October 2017, using quantitative real-time polymerase chain reaction method and newly designed specific primer-probe sets. Its abundances were >0.1 cells mL−1 at eight stations in January and March 2016 and March 2017, and its highest abundance in Korean waters was 26 cells mL−1. Thus, S. lachrymosa has a nationwide distribution in Korean waters as motile cells.
INTRODUCTIONDinoflagellates are known as one of the major components of diverse marine ecosystems (Wang et al. 2006, Jeong et al. 2013, Kang et al. 2013, Lim et al. 2017, Montero et al. 2017). They often form red tides or harmful algal blooms that sometimes cause human illness and large-scale mortality of fin-fish and shellfish (Shumway and Cembella 1993, Glibert et al. 2005, Jeong et al. 2010, 2015, 2017, Anderson et al. 2012, Park et al. 2013b). Thus, the presence of a dinoflagellate in the waters of a country that has large aquaculture and tourism industries is of critical concern to scientists, government officials, industry stakeholders, and public.
The genus Scrippsiella was first established by Balech (1959) and modified by Loeblich (1965). Later, more than 22 new species have been described in this genus (Gómez 2003, Gottschling et al. 2005, Luo et al. 2016). The species in this genus have been allocated to the subfamily Calciodinelloideae, because they have a calcareous layer within the wall of the resting cyst (Fensome et al. 1993, Head et al. 2006). Scrippsiella lachrymosa was first reported by Lewis (1991). Cysts of this species has been found in the sediments of several European countries, such as United Kingdom, Scotland, Greece, Italy, Germany, Spain, Portugal, Sweden, and Norway, as well as Canada, United States, Chile, and southern Benguela (Lewis 1991, Olli and Anderson 2002, Gómez 2003, Hoppenrath 2004, Gottschling et al. 2005, Joyce et al. 2005, Gottschling and Kirsch 2009, Pitcher and Joyce 2009, Satta et al. 2010, Zinssmeister et al. 2011, Soehner et al. 2012). In addition, the presence of S. lachrymosa in China was reported using internal transcribed spacer (ITS) ribotyping method (Zinssmeister et al. 2011, Soehner et al. 2012). Prior to the present study, only two studies (Kuylenstierna and Karlson 2000, Hoppenrath 2004) have reported the presence of motile cells of S. lachrymosa, which were found in net samples collected from the Helgoland Reede station, Germany, and Skagerrak and Kattegat, Sweden. Thus, it is worth investigating whether motile cells of this species exist in waters other than these northwestern European waters.
The overall morphology of Scrippsiella lachrymosa was first described by Lewis (1991). However, this study mentioned that the number of the sulcal plates in S. lachrymosa was questionable. Thus, in the present study, we analyzed the detailed morphology of the S. lachrymosa strain isolated from waters off Busan, Korea, in 2017, following which, its clonal culture was setup and the plates were carefully examined using scanning electron microscopy. Furthermore, using this culture, we analyzed the small subunit (SSU), ITS, and large subunit (LSU) rDNA sequences of this species, which were then compared with the LSU and ITS rDNA sequences of other S. lachrymosa strains (Zinssmeister et al. 2011, Soehner et al. 2012).
Morphological differences in motile forms of the species in genus Scrippsiella are smaller compared with other dinoflagellate genera (Lewis 1991, D’Onofrio et al. 1999, Meier et al. 2002, Gottschling et al. 2005, Zinssmeister et al. 2011). Thus, it is very difficult to distinguish one Scrippsiella species from another species in fixed samples. In order to quantify the abundance of S. lachrymosa in water samples, molecular techniques such as quantitative real-time polymerase chain reaction (qPCR) are needed. We designed a S. lachrymosa-specific primer-probe set, using which, we quantified the abundance of S. lachrymosa in the waters from 28 stations, located in the East, West, and South Sea of Korea during four seasons in 2016–2017. The results of the present study provide a basis for understanding the morphological and genetic characterizations of S. lachrymosa and its nationwide distribution.
MATERIALS AND METHODSCollection and culture of Scrippsiella lachrymosaThe Korean strain of S. lachrymosa (SLBS1703) was isolated from the surface water off Busan, southern Korea, in March 2017, when the water temperature and salinity were 10.9°C and 33.5, respectively (Fig. 1). A clonal culture of this species was established by two serial isolations. The culture was maintained in f/2 seawater medium (Guillard and Ryther 1962) at 20°C, with illumination of 20 μE m−2 s−1 cool white fluorescent light under a 14 : 10 h light-dark cycle. When the culture became dense, it was transferred every 2–3 weeks to a new 800-mL polystyrene culture flask containing f/2 seawater medium. This culture was then used to perform the genetic and morphological analyses.
MorphologyThe overall shape and size of living cells and cysts of the Korean strain of S. lachrymosa (SLBS1703) were analyzed using light microscopy. Furthermore, detailed morphological characterizations, such as the shape and size of each plate, and the number of plates, were analyzed using scanning electron microscopy (SEM). For SEM observation, cells from a dense culture of the Korean strain of S. lachrymosa were fixed for 10 min in paraformaldehyde at a final concentration of 2% (v/v) in filtered seawater. All other methods for SEM observation, including cell collection, dehydration, drying, and observation, were performed as described in Kang et al. (2010).
Nucleic acid extraction, polymerase chain reaction (PCR) amplification, sequencing, and phylogenetic analysesFor PCR amplification, nucleic acids were extracted from 10 to 15 mL of a dense culture of the Korean S. lachrymosa strain using the AccuPrep Genomic DNA Extraction Kit (Bioneer Cooperation, Daejeon, Korea), following manufacturer’s instructions. The PCR amplification for the SSU, LSU, and ITS region of the ribosomal DNA (rDNA) was performed using EukA, EukB (Stoeck et al. 2005), Euk1209F (Giovannoni et al. 1988), ITSR2 (Litaker et al. 2003), D1RF (Scholin et al. 1994), and LSUB (Litaker et al. 2003) (Table 1). Next, Solg f-Taq DNA Polymerase (SolGent Co., Daejeon, Korea) was used according to the manufacturer’s instructions to amplify the DNA. The DNA was amplified in a Mastercycler ep gradient (Eppendorf, Hamburg, Germany) using the following cycling conditions: 3 min at 94°C pre-denaturation, followed by 35 cycles of 1 min at 94°C, 1 min at the annealing temperature, and 2 min at 72°C, with a final extension of 5 min at 72°C.
The obtained sequences were manually edited using Contig Express (Invitrogen, Carlsbad, CA, USA). Next, phylogenetic analysis of the ITS regions of the Korean strain of S. lachrymosa was conducted using MEGA v.4 (Tamura et al. 2007), with the sequences of outgroup and other Scrippsiella spp. obtained from NCBI and recently published results (Zinssmeister et al. 2011, Soehner et al. 2012). Maximum likelihood (ML) analyses were conducted using the RAxML 7.0.4 program with a GTRGAMMA model (Stamatakis 2006). Further, 200 independent tree inferences were used to identify the best tree. ML bootstrap values were determined using 1,000 replicates. Bayesian analyses were conducted using MrBayes v.3.1 (Ronquist and Huelsenbeck 2003) in the default GTR + G + I model to determine the best available model for the data from each region. For all sequence regions, four independent Markov Chain Monte Carlo runs were performed, as described in Kang et al. (2010).
Design of TaqMan probe and primer setUsing the program MEGA v.4, ITS sequences of the Korean strain of S. lachrymosa obtained from PCR amplification were aligned with ITS rDNA sequences of the other strains of S. lachrymosa and related dinoflagellates, available from GenBank (Tamura et al. 2007). Manual searches of the alignments were conducted to determine unique sequences and to develop a S. lachrymosa specific primer-probe set for qPCR assay. The sequences for the primer-probe set were selected from the regions that were conserved within S. lachrymosa strains, but allowed discrimination with other dinoflagellates. The primer and probe sequences of the target species were analyzed with Primer 3 (Rozen and Skaletsky 2000) and Oligo Calc: Oligonucleotide Properties Calculator (Kibbe 2007) software for optimal melting temperature and secondary structure. Subsequently, the primers and probe were synthesized by Biosearch Technologies (Petaluma, CA, USA). The probe was dual-labeled with the fluorescent dyes, FAM and BHQplus (Biosearch Technologies Inc., Novato, CA, USA), at the 5′ and 3′ ends, respectively (Table 1).
Quantifying the abundance of Scrippsiella lachrymosa in the Korean watersUsing a clean bucket, water samples were collected from the surface of 28 stations in East, West, and South Sea of Korea in January, March, July, October, and December in 2016 and March, July, and October in 2017 (Fig. 1).
Samples for qPCR (50–300 mL) were collected, filtered through 25-mm GF/C filter (Whatman Inc., Floreham Park, NJ, USA). The filter was loosely rolled and placed into a 1.5-mL tube and frozen under −20°C until transfer to the laboratory. The DNA from cells on the filter of each sample was extracted as described above.
Generating standard curve and qPCR amplificationTo obtain the standard curve, DNA was extracted from a dense clonal culture of S. lachrymosa, targeting 100,000 cells in the final elution volume of 100 μL, using the identical method mentioned above. The extracted DNA was then serially diluted by adding predetermined volumes of deionized sterile water (DDW) (Bioneer Cooperation) to the 1.5-mL tubes, to ultimately prepare 6 different DNA concentrations, targeting 100, 10, 1, 0.1, 0.01, and 0.001% DNA concentration of the originally extracted DNA. Then samples were stored at −20°C in the freezer and qPCR amplification was conducted within a day. The qPCR assays for determination of the standard curve were performed using the following steps modified from Lee et al. (2017): 1 μL of DNA template, 0.2 μM of primers (forward and reverse) and 0.15 μM of probe (final concentrations), and 5 μL of HiFast Probe Hi-Rox (Genepole, Gwangmyung, Korea) were combined and DDW was added to each sample, resulting in the total final volume of 10 μL. The qPCR analyses were performed using Rotor-Gene Q (Qiagen, Hilden, Germany) under following thermal cycling conditions: 3 min at 95°C, followed by 40 cycles of 10 s at 95°C, and 40 s at 58°C.
The aforementioned qPCR assay conditions were used to determine the abundance of S. lachrymosa in field samples. The DNA from each sample was amplified four times to ensure the accuracy of results. Samples using DDW as the template were used as the negative control, whereas the DNA used to construct standard curve was used as positive and standard control.
RESULTSMorphology of the Korean strain of Scrippsiella lachrymosaUnder an inverted microscope, motile cells of the Korean S. lachrymosa strain were observed to be conical in epitheca, and rounded in hypotheca (Fig. 2A & B). The cell length and width of the photosynthetically grown motile cells (n = 20) were 16.3–23.2 and 13.0–21.5 μm, respectively (Table 2). In addition, the length-to-width ratio of the motile cells was 1.0–1.5 (Table 2). However, when fixed and observed under SEM, the cell length and width were 14.6–21.2 and 12.3–18.1 μm, respectively (Table 2). The ratio of the length to the width under SEM was 1.0–1.3. In addition, the shape of non-motile coccoid cells (cysts) was oval (Fig. 2C & D). When observed under an inverted microscope, the length and width of the coccoid cells (n = 20) were found to be 17.6–29.4 and 12.3–21.6 μm, respectively (Table 2). The length-to-width ratio of the coccoid cells was 1.0–1.7.
When observed under SEM, the shape of epitheca of motile cells of the Korean S. lachrymosa strain was conical, whereas hypotheca was rounded (Figs 3A, C–E, 4A & B). The apical horn was of smooth circular-ridged shape, with upright collar and narrow intercalary bands around the pore plate (Po) located at the top (Figs 3A–E & 4A–C). The collar was extended to the narrow canal plate (x), which touched the 1′, 2′, and 4′ plates. The 1′ plate was narrow and hexagonal, and the 2′ plate was hexagonal, touching the x, Po, 1′, 3′, 1″, 2″, and 1a plates. The hexagonal arch-shaped 3′ plate surrounded the Po plate and touched the 2′ and 4′ plates as well as the 1a-3a plates, whereas the hexagonal 4′ plate touched the x, Po, 1′, 3′, 6″, 7″, and 3a plates (Figs 3A–E & 4A–C). There were three intercalary plates, including pentagonal 1a, hexagonal 2a, and pentagonal 3a plates (Figs 3B–E & 4A–C). The 1a plate touched the 2′, 3′, 2″, 3″, and 2a plates, whereas the 2a plate touched the 3′, 3″, 4″, 5″, 1a, and 3a plates (Figs 3B–E, 4B & C). The 3a plate touched the 3′, 4′, 5″, 6″, and 2a plates (Figs 3B, E & 4A–C).
The S. lachrymosa cells had seven precingular plates: quadrangular 1″, 4″, 7″ plates and pentagonal 2″, 3″, 5″, 6″ plates (Table 2, Figs 3A–E & 4A–C). Furthermore, S. lachrymosa had one cingulum row, comprising six rectangular-shaped cingulum plates located in the center of the cell (Figs 3A, C–E, G, 4A & B). The cingulum was displaced by 0.08–0.19 times the cell length and 0.13–0.22 times the cell width (n = 20).
There were six postcingular plates (Table 2, Figs 3C–G & 4D). The 1‴, 2‴, 4‴, and 5‴ plates were quadrangular, whereas the 3‴ plate was pentagonal. In addition, there were two antapical plates, which were pentagonal (Table 2, Figs 3F, G, 4D & 5D). The 1⁗ plate touched the 1‴, 2‴, 3‴, and 2⁗ plates along with the posterior sulcal (s.p.) plate, whereas the 2⁗ plate touched the 3‴, 4‴, 5‴, 1⁗, and s.p. plates (Figs 3F, G, 4D & 5D). Furthermore, the Korean S. lachrymosa strain had five sulcal plates: the anterior sulcal plate (s.a.), right sulcal plate (s.d.), left sulcal plate (s.s.), median sulcal plate (s.m.), and s.p. plate (Figs 3A, F, 4A & 5).
Molecular characterization of the Korean strain of Scrippsiella lachrymosaWhen compared, the sequence of LSU rDNA of the Korean strain of S. lachrymosa (SLBS1703) was different by 5 bp (0.7–0.8%) from those of two Norwegian strains of S. lachrymosa, the only strains for which LSU sequences have been reported. In addition, the sequence of LSU rDNA of S. lachrymosa SLBS1703 was 31 bp (4.6%) different from S. trifida, which is known to be morphologically similar to S. lachrymosa (Table 3). Furthermore, the sequence of the ITS regions of S. lachrymosa SLBS1703 was 1–27 bp (0.4–6.0%) different from those of the other reported strains of S. lachrymosa, and 75–88 bp (16.1–16.2%) different from those of two S. trifida strains (Table 4).
In the phylogenetic tree based on LSU rDNA sequences of Scrippsiella spp., the Korean strain of S. lachrymosa formed a clade with two reported Norwegian strains (Fig. 6). Moreover, in the phylogenetic tree based on ITS rDNA sequences, the Korean strain of S. lachrymosa also formed a clade with the Chinese (42D4) and Scottish (42E5) strains, but the clade was clearly divergent from the clade with the Norwegian (GeoB 259 and GeoB 288) and Canadian (GeoB 341 and GeoB 344) strains (Fig. 7).
Distribution of Scrippsiella lachrymosa in Korean water using qPCRAmong the 28 stations investigated in this study, S. lachrymosa was found in the waters of eight stations (i.e., Uljin, Pohang, Busan, Dadaepo, Jinhae, Kunsan, Buan, and Mokpo) in January and March 2016, and March 2017. The highest abundance of S. lachrymosa, 26.3 cells mL−1, was obtained in Jinhae in March 2016, whereas the second highest abundance, 6.2 cells mL−1, was obtained in Mokpo in March 2017 (Table 5, Fig. 1). The ranges of the temperature and salinity of the waters at the stations where S. lachrymosa was detected were 5.4–14.9°C and 30.5–34.3, respectively, while those at all stations were 1.1–28.0°C and 9.6–35.6, respectively (Table 6).
DISCUSSIONMorphology of the Korean strain of Scrippsiella lachrymosaThe overall morphology of the Korean strain of S. lachrymosa looks similar to that of the Scottish strain (Lewis 1991). However, there are some morphological differences between these two strains (Table 2). First, the 2′, 3′, and 4′ apical plates of the Korean strain are hexagonal, whereas those of the Scottish strain are all pentagonal. Second, the size of cysts of the Korean strain is 17.6–29.4 μm, considerably smaller than that of the Scottish strain (34–44 μm). Thus, the detailed morphology of the Korean strain of S. lachrymosa is somewhat different from that of the Scottish strain. Lewis (1991) did not provide the exact number of the sulcal plates of the Scottish strain. The results of this study clearly show that the Korean strain of S. lachrymosa has five sulcal plates (i.e., s.a., s.d., s.s., s.p., and s.m.). Thus, the plate formula of the Korean strain of S. lachrymosa can be completely determined as Po, x, 4′, 3a, 7″, 6c, 5s, 5‴, and 2⁗.
Molecular characterization of the Korean strain of Scrippsiella lachrymosaThe sequences of both LSU and ITS rDNA of the Korean S. lachrymosa strain are different from those of any other strain of S. lachrymosa. Therefore, the Korean strain of S. lachrymosa has a unique sequence.
The LSU rDNA sequence of the Korean strain of S. lachrymosa is slightly different from that of the Norwegian strain, and in the phylogenetic tree based on the sequences of the LSU of Scrippsiella spp., the Korean strain forms a clade with these Norwegian strains. However, in the phylogenetic tree based on the sequences of the ITS of Scrippsiella spp., the Korean strain of S. lachrymosa belongs to a clade that is divergent from the clade containing these Norwegian strains. Therefore, the Korean strain of S. lachrymosa is clearly genetically different from the Norwegian strains. There are only 3 strains of S. lachrymosa whose LSU sequences have been reported, whereas there are 15 strains of S. lachrymosa whose ITS sequences have been reported. Therefore, the lack of LSU sequences may have resulted in the formation of the clade comprising the Korean and Norwegian strains.
Lewis (1991) reported that the motile form of S. lachrymosa is morphologically similar to S. trifida. The LSU and ITS sequences of the Korean S. lachrymosa strain are considerably different from those of S. trifida, and in the LSU and ITS phylogenetic trees, the clades containing the Korean strain are clearly divergent from those containing S. trifida. Therefore, the results of the present study confirm that these two are different species.
Distribution of Scrippsiella lachrymosa in Korean watersWe report for the first time, the occurrence of S. lachrymosa in Korean waters. So far, the presence of S. lachrymosa has been reported mostly in the waters of European countries and rarely in America, Africa, and Asia (Lewis 1991, Nehring 1994, Gómez 2003, Hoppenrath 2004, Gottschling et al. 2005, Joyce et al. 2005, Gottschling and Kirsch 2009, Pitcher and Joyce 2009, Satta et al. 2010, Zinssmeister et al. 2011, Soehner et al. 2012). Thus, the results of this study add Korean waters to the locations where S. lachrymosa are present.
S. lachrymosa is one of the common resting cyst-producing dinoflagellates (e.g., Persson et al. 2016). The cultures of the other stains of S. lachrymosa were established from cysts in the sediments (Olli and Anderson 2002, Olli et al. 2004, Persson et al. 2016), but the clonal culture of the Korean strain of S. lachrymosa was established from motile cells. Olli and Anderson (2002) reported that the dormancy period of the newly formed S. lachrymosa cysts was approximately 2 months. Thus, the environmental conditions under which the cysts of S. lachrymosa form could be different from those under which motile cells of S. lachrymosa are present. Therefore, to investigate the effects of environmental factors on distributions of S. lachrymosa, environmental conditions under which motile cells of S. lachrymosa occur should be understood. Kuylenstierna and Karlson (2000) and Hoppenrath (2004) reported the presence of motile S. lachrymosa cells in net samples collected from northwestern European waters, but they did not provide data on the environmental conditions. Therefore, the present study provides data about the environmental conditions under which motile cells of S. lachrymosa exist, which would help understand the ecophysiology of this species.
S. lachrymosa was found in eight locations along the Korean peninsula. Thus, S. lachrymosa has a nationwide distribution in Korea. The Korean stations where S. lachrymosa was found are located at the latitudes of 35°07′-36°54′ N. The latitudes at which the other strains of S. lachrymosa were found are 27°12′-63°42′ N (Zinssmeister et al. 2011, Soehner et al. 2012). Thus, S. lachrymosa may have a wide latitudinal distribution.
During this study, the abundances of S. lachrymosa with >0.1 cells mL−1 were observed in January and March 2016 and in March 2017, when the water temperature was 5.4–14.9°C. Thus, the Korean strain of S. lachrymosa is likely to have a seasonality. Meanwhile, most of the S. lachrymosa cysts collected in the European countries were found when the water temperatures were 18–20°C (Rubino et al. 2010). However, these water temperatures may not be a good representative of the water temperatures at which motile cells of S. lachrymosa exist. Therefore, to determine whether the European strains have a seasonality or not, the distribution of motile cells with ambient water temperatures should be explored.
The highest abundance of S. lachrymosa in the Korean waters in 2016–2017 was 26 cells mL−1. The present study for the first time reports the abundance of motile S. lachrymosa. The highest abundance of the red-tide dinoflagellate Scrippsiella acuminata (previously S. trochoidea) is ca. 15,000 cells mL−1 (Park et al. 2013a). Although the highest abundance of S. lachrymosa during the study period is much lower than that of S. acuminata in Korean waters, with additional sampling, the highest abundance of S. lachrymosa may increase in the Korean waters.
ACKNOWLEDGEMENTSWe thank Kyung Ha Lee, Jin Hee Ok, An Suk Lim, Hee Chang Kang, Se Hyeon Jang, Ji Eun Kwon, Jae Yeon Park, and Eun Young Yoon, for technical support. This research was supported by the Useful Dinoflagellate program of Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) and Management of marine organisms causing ecological disturbance and harmful effect Program of KIMST, the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2015M1A5A1041806;NRF-2017R1E1A1A01074419) award to HJJ.
Fig. 1Map of the sampling stations of the study area in Korea. The circles indicate the sampling stations. The closed circles indicate the stations at which the abundance of Scrippsiella lachrymosa was >0.1 cells mL−1. The numbers in the parenthesis are the highest abundance at a station and the date when the highest abundance was obtained at the station. SC, Sokcho; JMJ, Jumunjin; DH, Donghae; UJ, Uljin; PH, Pohang; US, Ulsan; BS, Busan; DDP, Dadaepo; MS, Masan; JH, Jinhae; TY, Tongyoung; YS, Yeosu; KY, Kwangyang; GH, Goheung; JAH, Jangheung; AS, Ansan; DAJ, Dangjin; MGP, Mageompo; SCN, Seocheon; KS, Kunsan; BA, Buan; MP, Mokpo; AW, Aewol; GS, Gosan; SGP, Seogwipo; WM, Wimi; SS, Seongsan; GN, Gimnyeong. 
Fig. 2Micrographs of motile cells and non-motile coccoid cells (cysts) from the clonal culture of Scrippsiella lachrymosa SLBS1703, obtained by light microscopy. (A & B) Motile cells. (C & D) Non-motile coccoid cells. Scale bars represent: A–D, 10 μm. 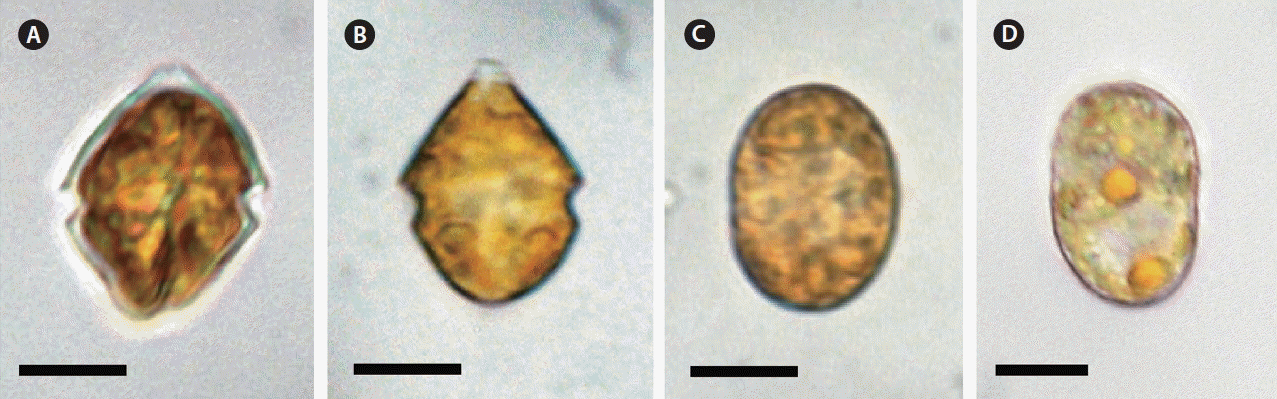
Fig. 3Micrographs of motile cells of Scrippsiella lachrymosa obtained by scanning electron microscopy. (A) Ventral view, showing the episome, cingulum (c), sulcus (s.a., s.d., s.s., s.m., and s.p.), and hyposome. (B) Apical view, showing the episome and Po plate. (C) Ventral-left lateral view, showing the episome, cingulum (c), and hyposome. (D) Dorsal view, showing the episome, cingulum (c), and hyposome. (E) Ventral-right lateral view, showing the episome, cingulum (c), and hyposome. (F) Antapical view, showing the hyposome, and sulcus (s.a., s.d., s.s., s.p., and s.m.). (G) Antapical-dorsal view, showing the cingulum (c), sulcus (s.p.), and hyposome. s.a., anterior sulcal plate; s.d., right sulcal plate; s.s., left sulcal plate; s.m., median sulcal plate; s.p., posterior sulcal plate. Scale bars represent: A–G, 2 μm. 
Fig. 4Drawings of motile cells of Scrippsiella lachrymosa, showing the external morphology. (A) Ventral view. (B) Dorsal view. (C) Apical view. (D) Antapical view. s.a., anterior sulcal plate; s.d., right sulcal plate; s.s., left sulcal plate; s.m., median sulcal plate; s.p., posterior sulcal plate. Scale bars represent: A–D, 2 μm. 
Fig. 5Micrographs of sulcal plates in motile cells of Scrippsiella lachrymosa, obtained by scanning electron microscopy. (A) Ventral view of the sulcus (s.a., s.d., s.s., s.p., and s.m.) and cingulum (c). (B) Ventral right view of the sulcus. (C) Ventral left view of the sulcus. (D) Antapical-dorsal view of the sulcus (s.a., s.d., s.s., s.p., and s.m.). (E) Enlarged view from (D) showing the sulcus. s.a., anterior sulcal plate; s.d., right sulcal plate; s.s., left sulcal plate; s.m., median sulcal plate; s.p., posterior sulcal plate. Scale bars represent: A–E, 2 μm. 
Fig. 6Consensus Bayesian tree based on 610 bp aligned positions of the large subunit regions, using the GTR + G + I model and Cryptoperidiniopsis brodyi as an outgroup taxa. The parameters were as follows: assumed nucleotide frequencies as equal, substitution rate matrix with A–C substitutions = 0.0320, A–G substitutions = 0.1515, A–T substitutions = 0.0521, C–G substitutions = 0.0136, C–T substitutions = 0.6791, and G–T substitutions = 0.0717, proportion of sites assumed to be invariable = 0.2699, and the rates for variable sites assumed to follow a gamma distribution with shape parameter = 0.5103. The branch lengths are proportional to the amount of character changes. The numbers above the branches indicate the Bayesian posterior probability (left) and maximum likelihood bootstrap values (right). Posterior probabilities ≥ 0.5 are shown. 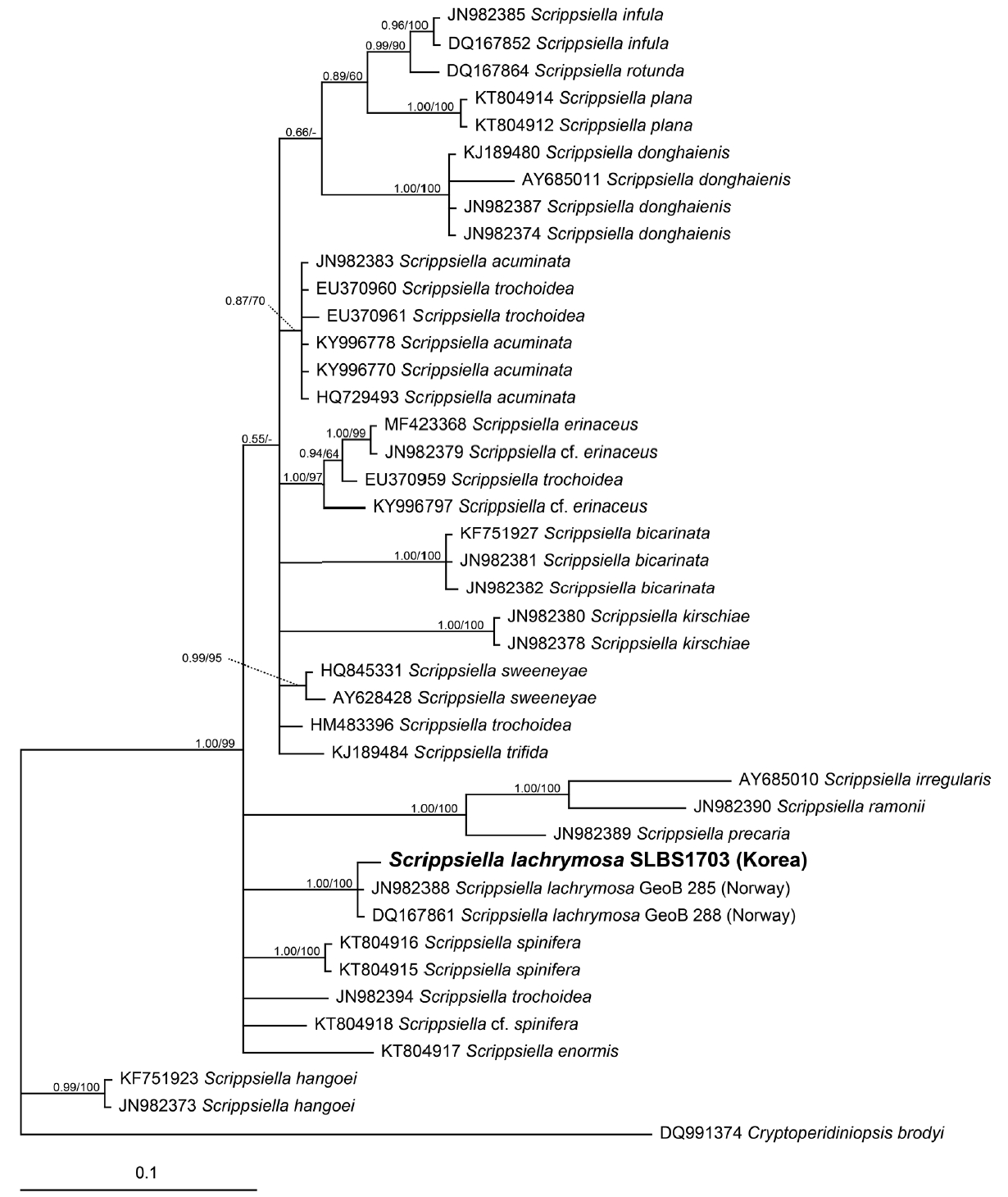
Fig. 7Consensus Bayesian tree based on 695 bp aligned positions of the internal transcribed spacer regions, using the GTR + G + I model and Heterocapsa triquetra as an outgroup taxa. The parameters were as follows: assumed nucleotide frequencies as equal, substitution rate matrix with A–C substitutions = 0.0944, A–G substitutions = 0.2959, A–T substitutions = 0.1035, C–G substitutions = 0.0524, C–T substitutions = 0.3634, and G–T substitutions = 0.0904, proportion of sites assumed to be invariable = 0.1109, and rates for variable sites assumed to follow a gamma distribution with shape parameter = 0.6460. The branch lengths are proportional to the amount of character changes. The numbers above the branches indicate the Bayesian posterior probability (left) and maximum likelihood bootstrap values (right). Posterior probabilities ≥ 0.5 are shown. 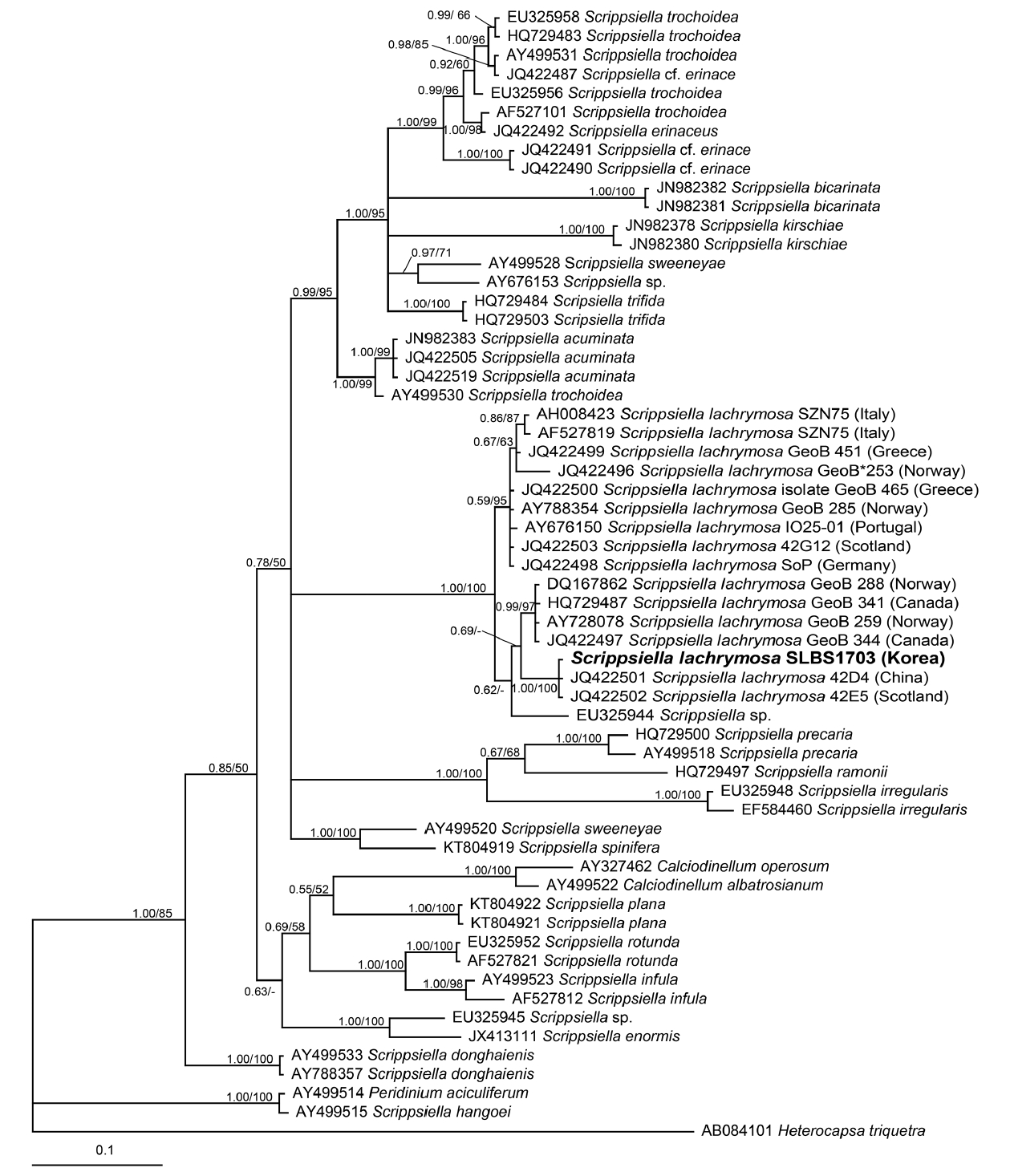
Table 1Oligonucleotide primers used to amplify the SSU, ITS, and LSU regions of ribosomal DNA and the species-specific primers and TaqMan probes used to determine the abundance of Scrippsiella lachrymosa using qPCR method
Table 2Comparison of the morphology of the Korean strain of Scrippsiella lachrymosa and other Scrippsiella species based on specimens observed by light microscopy (LM) and scanning electron microscopy (SEM)
Table 3Comparison of the sequences of the large subunit ribosomal DNA of the strains of Scrippsiella lachrymosa and Scrippsiella trifida, which are known to be morphologically similar to each other
Table 4Comparison of the sequences of the internal transcribed spacer ribosomal DNA of the strains of Scrippsiella lachrymosa (SL) and Scrippsiella trifida
Table 5The abundance of Scrippsiella lachrymosa (cells mL−1) in the waters of the stations along the Korean coasts, measured using qPCR method Table 6Temperature and salinity of the waters collected from the stations in the study period (January 2016 to October 2017)
Tall, range of temperatures of the waters collected from all stations; Td, range of temperatures of the waters collected at the stations where Scrippsiella lachrymosa was found; Sall, range of salinities of the waters collected from all stations; Sd, range of salinities of the waters collected at the stations where S. lachrymosa was found. REFERENCESAnderson, DM., Alpermann, TJ., Cembella, AD., Collos, Y., Masseret, E. & Montresor, M. 2012. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 14:10–35.
D’Onofrio, G., Marino, D., Bianco, L., Busico, E. & Montresor, M. 1999. Toward an assessment on the taxonomy of dinoflagellates that produce calcareous cysts (Calciodinelloideae, Dinophyceae): a morphological and molecular approach. J Phycol. 35:1063–1078.
Fensome, RA., Taylor, FJR., Norris, G., Sarjeant, WAS., Wharton, DI. & Williams, GL. 1993. A classification of living and fossil dinoflagellates. Micropaleontology. Special Publication No. 7. Sheridan Press, Hanover, PA, 351 pp.
Giovannoni, SJ., DeLong, EF., Olsen, GJ. & Pace, NR. 1988. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 170:720–726.
Glibert, PM., Anderson, DM., Gentein, P., Granéli, E. & Sellner, KG. 2005. The global, complex phenomena of harmful algal blooms. Oceanography. 18:136–147.
Gottschling, M. & Kirsch, M. 2009. Annotated list of Scandinavian calcareous dinoflagellates collected in fall 2003. Berl Paläobiologische Abh. 10:193–198.
Gottschling, M., Knop, R., Plötner, J., Kirsch, M., Willems, H. & Keupp, H. 2005. A molecular phylogeny of Scrippsiella sensu lato (Calciodinellaceae, Dinophyta) with interpretations on morphology and distribution. Eur J Phycol. 40:207–220.
Guillard, RRL. & Ryther, JH. 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol. 8:229–239.
Head, MJ., Lewis, J. & de Vernal, A. 2006. The cyst of the calcareous dinoflagellate Scrippsiella trifida: resolving the fossil record of its organic wall with that of Alexandrium tamarense
. J Paleontol. 80:1–18.
Hoppenrath, M. 2004. A revised checklist of planktonic diatoms and dinoflagellates from Helgoland (North Sea, German Bight). Helgol Mar Res. 58:243–251.
Jeong, HJ., Lim, AS., Franks, PJS., Lee, KH., Kim, JH., Kang, NS., Lee, MJ., Jang, SH., Lee, SY., Yoon, EY., Park, JY., Yoo, YD., Seong, KA., Kwon, JE. & Jang, TY. 2015. A hierarchy of conceptual models of red-tide generation: nutrition, behavior, and biological interactions. Harmful Algae. 47:97–115.
Jeong, HJ., Lim, AS., Lee, K., Lee, MJ., Seong, KA., Kang, NS., Jang, SH., Lee, KH., Lee, SY., Kim, MO., Kim, JH., Kwon, JE., Kang, HC., Kim, JS., Yih, W., Shin, K., Jang, PK., Ryu, J-H., Kim, SY., Park, JY. & Kim, KW. 2017. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: I. Temporal variations in three-dimensional distributions of red-tide organisms and environmental factors. Algae. 32:101–130.
Jeong, HJ., Yoo, YD., Kim, JS., Seong, KA., Kang, NS. & Kim, TH. 2010. Growth, feeding, and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J. 45:65–91.
Jeong, HJ., Yoo, YD., Lee, KH., Kim, TH., Seong, KA., Kang, NS., Lee, SY., Kim, JS., Kim, S. & Yih, W. 2013. Red tides in Masan Bay, Korea in 2004–2005, I. Daily variations in the abundance of red-tides organisms and environmental factors. Harmful Algae. 30(Suppl 1):S75–S88.
Joyce, LB., Pitcher, GC., Du Randt, A. & Monteiro, PMS. 2005. Dinoflagellate cysts from surface sediments of Saldanha Bay, South Africa: an indication of the potential risk of harmful algal blooms. Harmful Algae. 4:309–318.
Kang, NS., Jeong, HJ., Moestrup, Ø., Shin, W., Nam, SW., Park, JY., de Salas, MF., Kim, KW. & Noh, JH. 2010. Description of a new planktonic mixotrophic dinoflagellate Paragymnodinium shiwhaense n. gen., n. sp. from the coastal waters off western Korea: morphology, pigments, and ribosomal DNA gene sequence. J Eukaryot Microbiol. 57:121–144.
Kang, NS., Lee, KH., Jeong, HJ., Yoo, YD., Seong, KA., Potvin, É., Hwang, YJ. & Yoon, EY. 2013. Red tides in Shiwha Bay, western Korea: a huge dike and tidal power plant established in a semi-enclosed embayment system. Harmful Algae. 30(Suppl 1):S114–S130.
Kibbe, WA. 2007. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 35(Suppl 2):W43–W46.
Kuylenstierna, M. & Karlson, B. 2000. Checklist of phytoplankton in Skagerrak-Kattegat. Available from: http://www.smhi.se/oceanografi/oce_info_data/plankton_checklist/ssshome.htm
. Accessed Feb 28, 2018
Lee, SY., Jeong, HJ., Seong, KA., Lim, AS., Kim, JH., Lee, KH., Lee, MJ. & Jang, SH. 2017. Improved real-time PCR method for quantification of the abundance of all known ribotypes of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides by comparing 4 different preparation methods. Harmful Algae. 63:23–31.
Lewis, J. 1991. Cyst-theca relationships in Scrippsiella (Dinophyceae) and related orthoperidinioid genera. Bot Mar. 34:91–106.
Lim, AS., Jeong, HJ., Seong, KA., Lee, MJ., Kang, NS., Jang, SH., Lee, KH., Park, JY., Jang, TY. & Yoo, YD. 2017. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: II. Heterotrophic protists and their grazing impacts on red-tide organisms. Algae. 32:199–222.
Litaker, RW., Vandersea, MW., Kibler, SR., Reece, KS., Stokes, NA., Steidinger, KA., Millie, DF., Bendis, BJ., Pigg, RJ. & Tester, PA. 2003. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J Phycol. 39:754–761.
Luo, Z., Mertens, KN., Bagheri, S., Aydin, H., Takano, Y., Matsuoka, K., McCarthy, FMG. & Gu, H. 2016. Cyst-theca relationship and phylogenetic positions of Scrippsiella plana sp. nov. and S. spinifera (Peridiniales, Dinophyceae). Eur J Phycol. 51:188–202.
Meier, KJS., Janofske, D. & Willems, H. 2002. New calcareous dinoflagellates (Calciodinelloideae) from the Mediterranean Sea. J Phycol. 38:602–615.
Montero, P., Pérez-Santos, I., Daneri, G., Gutiérrez, MH., Igor, G., Seguel, R., Purdie, D. & Crawford, DW. 2017. A winter dinoflagellate bloom drives high rates of primary production in a Patagonian fjord ecosystem. Estuar Coast Shelf Sci. 199:105–116.
Nehring, S. 1994.
Scrippsiella spp. resting cysts from the German Bight (North Sea): a tool for more complete check-lists of dinoflagellates. Neth J Sea Res. 33:57–63.
Olli, K. & Anderson, DM. 2002. High encystment success of the dinoflagellate Scrippsiella cf. lachrymosa in culture experiments. J Phycol. 38:145–156.
Olli, K., Neubert, MG. & Anderson, DM. 2004. Encystment probability and encystment rate: new terms to quantitatively describe formation of resting cysts in planktonic microbial populations. Mar Ecol Prog Ser. 273:43–48.
Park, J., Jeong, HJ., Yoo, YD. & Yoon, EY. 2013a. Mixotrophic dinoflagellate red tides in Korean waters: distribution and ecophysiology. Harmful Algae. 30(Suppl 1):S28–S40.
Park, TG., Lim, WA., Park, YT., Lee, CK. & Jeong, HJ. 2013b. Economic impact, management and mitigation of red tides in Korea. Harmful Algae. 30(Suppl 1):S131–S143.
Persson, A., Smith, BC., Cyronak, T., Cooper, E. & DiTullio, GR. 2016. Differences in pigmentation between life cycle stages in Scrippsiella lachrymosa (dinophyceae). J Phycol. 52:64–74.
Pitcher, GC. & Joyce, LB. 2009. Dinoflagellate cyst production on the southern Namaqua shelf of the Benguela upwelling system. J Plankton Res. 31:865–875.
Ronquist, F. & Huelsenbeck, JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574.
Rozen, S. & Skaletsky, H. 2000. Primer3 on the WWW for general users and for biologist programmers. In : Misener S, Krawetz SA, editors Bioinformatics Methods and Protocols. Methods in Molecular Biology. Humana Press, Totowa, NJ, 365–386.
Rubino, F., Belmonte, M., Caroppo, C. & Giacobbe, M. 2010. Dinoflagellate cysts from surface sediments of Syracuse Bay (Western Ionian Sea, Mediterranean). Deep-Sea Res Part II Top Stud Oceanogr. 57:243–247.
Satta, CT., Anglès, S., Garcés, E., Lugliè, A., Padedda, BM. & Sechi, N. 2010. Dinoflagellate cysts in recent sediments from two semi-enclosed areas of the Western Mediterranean Sea subject to high human impact. Deep Sea Res Part II Top Stud Oceanogr. 57:256–267.
Scholin, CA., Herzog, M., Sogin, M. & Anderson, DM. 1994. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J Phycol. 30:999–1011.
Shumway, SE. & Cembella, AD. 1993. The impact of toxic algae on scallop culture and fisheries. Rev Fish Sci. 1:121–150.
Soehner, S., Zinssmeister, C., Kirsch, M. & Gottschling, M. 2012. Who am I-and if so, how many? Species diversity of calcareous dinophytes (Thoracosphaeraceae, Peridiniales) in the Mediterranean Sea. Org Divers Evol. 12:339–348.
Stamatakis, A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.
Stoeck, T., Schwarz, MVJ., Boenigk, J., Schweikert, M., von der Heyden, S. & Behnke, A. 2005. Cellular identity of an 18S rRNA gene sequence clade within the class Kinetoplastea: the novel genus Actuariola gen. nov. (Neobodonida) with description of the type species Actuariola framvarensis sp. nov. Int J Syst Evol Microbiol. 55:2623–2635.
Tamura, K., Dudley, J., Nei, M. & Kumar, S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599.
|
|