INTRODUCTION
The demand for oleaginous algae for the biofuel, pharmaceutical, and nutraceutical industries is growing. Mass production of algae is a superior alternative over traditional crop and residual feedstock for biodiesel and bioproducts since algae have high growth and oil-accumulation rates, can sequester carbon dioxide, and can thrive on non-arable land and wastewater (Borowitzka and Moheimani 2013). When algae do not produce the required biofuel lipid profile, high-valued lipid compounds have proven indispensable towards nutraceuticals, pharmaceuticals, and cosmeceuticals (Ward and Singh 2005). A wide range of algal-derived lipids are produced for use in health supplements, food and animal feed additives, and have an immeasurable potential for the treatment of numerous human conditions (Zhang et al. 2010, Costa et al. 2012, Gantar et al. 2012, Xia et al. 2013).
Algae are emerging as one of the most promising and sustainable sources of fatty acids, including omega-3 (ω-3) and omega-7 (ω-7), which are directly extracted and marketed as vegan and vegetarian alternatives. Omega-7 fatty acids, or palmitoleic acid (16:1n7; PA), may be beneficial to the nutraceutical industry for its favorable effects on human health. PA is a promising compound for managing metabolic syndrome, a collection of symptoms including central obesity and glucose and insulin resistance, which is currently treated using several drugs with the additional risk of side effects. Preclinical and epidemiological studies have shown that anti-inflammatory and lipid lowering effects of PA are linked to the prevention of metabolic syndrome, which is associated with the risk of developing cardiovascular disease and type 2 diabetes. It is reported that PA reduces high-sensitivity C-reactive protein, triglycerides and low-density lipoproteins, while increasing high-density lipoproteins (Bernstein et al. 2014, Morse 2015). Two major plant sources of PA include seeds of sea buckthorn (Hippophae rhamnoides L.) with 32% PA content and seeds of macadamia (Macadamia integrifolia Maiden et Betche) with 20 to 30% PA content (Saleeb et al. 1973, Yang and Kallio 2001). These plants are valuable sources with limited availability and therefore may not be sustainable if the demand for PA increases beyond the limited supply from plant sources.
Although the traditional extraction of PA from plants may not be sustainable, the effort to obtain PA from viable sources extends towards microalgae (Lenihan-Geels et al. 2013). Algae-derived PA can be achieved in larger quantities in shorter time frames than in plants by optimizing algal culture conditions (Zhu et al. 2016). The extraction of oils directly from the microalgae is superior over conventional methods since the main components of fish oil (ω-3 and ω-7) come from the algae in their diets (Ofosu et al. 2017). Extracting PA from algae avoids the disadvantages of using fish including metal contamination and the disagreeable taste and odor often associated with fish oils (Adarme-Vega et al. 2012). Moreover, sourcing omega fatty acids from fish may compete with global food supply, especially in a changing climate where fisheries’ productivity is challenged in sustained provision of stock (Core Writing Team et al. 2014). Overall, using algae as an alternative can supplement the current and emerging uses of ω-7 and help reduce the demand and burden on aquacultural and agricultural systems (Knothe 2010).
Although algae are a promising source of essential omega fatty acids, producing algae-derived biofuels and high value compounds is challenging considering the level of scaling-up required for mass production of high-quality product (Hannon et al. 2010). To achieve large-scale algae-based production of compounds, it is imperative to have control over optimal conditions for highest yield. Control over biological and physicochemical properties of algae culture systems is essential for identifying and overcoming barriers to the scaling-up process. The primary issue associated with the success of large-scale microalgae cultivation is contamination (McBride et al. 2014). Algal cultures can become contaminated with herbivores and algal pathogens (viruses, bacteria, and fungi) and the main strain can be outcompeted for resources by fungi, bacteria, microbes, and other microalgal strains (Shunyu et al. 2006). Biological contamination reduces productivity and often results in culture failures and consequent loss of product. Despite the form of cultivation, whether open raceways or photobioreactors, biological pollutants are introduced through water input and gas exchange (Wang et al. 2013). To avoid the time and cost expenditure associated with culture contaminations, methods of prevention and eradication without interrupting microalgae growth are desired (Scott et al. 2010).
A primary approach in facilitating high biomass and lipid productivity while preventing contamination involves regulating the physicochemical and biological parameters of cultivation. Physicochemical methods of purifying culture contamination involve altering culture temperature or chemical treatment with possible negative effects on the target culture (Moreno-Garrido and Cañavate 2001). Biological methods for preventing culture contamination include the application of polycultures of algae, allelopathy, or selecting and using extremophile strains capable of enduring harsh cultivation conditions. Polyculture approaches can be designed where microalgae communities, with the desired product, are able to resist contamination (Smith and Crews 2014). While polycultures are plausible, they are difficult to construct as random assemblages may introduce complexity (Weis et al. 2008). Contamination of algal cultures can be prevented by using strains that excrete secondary metabolites with inhibitory allelopathic effects against the contaminating organisms (Chiang et al. 2004). However, obtaining a strain that is both highly productive in terms of lipid accumulation with inhibitory effects on other algae is challenging.
Another approach to maintaining contaminant-free algal cultures is to use extremophiles capable of thriving in extreme pH, temperature or salinity conditions, where other organisms cannot (Barnard et al. 2010). To date, a limited number of extremophile algal species are available and used for commercial purposes as opposed to marine and freshwater algae (Mutanda et al. 2011). For example, Dunaliella salina (Dunal) Teodoresco is grown in open outdoor raceway systems in a medium with high salinity, while Spirulina is grown in a medium with high bicarbonate content and alkaline pH (Jin and Melis 2003, Gantar and Svirčev 2008). High halo-alkaline conditions not only restrict the persistence of contaminants and competing organisms, but may also generate triacylglycerol synthesis (Gardner et al. 2011). Furthermore, employing bicarbonate as a carbon source is superior to carbon dioxide injection as it is more soluble and is retained in the culture medium and not lost by outgassing (Chi et al. 2011). The use of carbonates in conjunction with steep alkaline conditions and competent oleaginous microalgae can provide an ideal setting for large-scale production of biofuels and compounds essential to human supplements and drugs (Wensel et al. 2014).
Although application of alkaliphilic or alkali-tolerant microalgae may be practical in mass cultivation, native lipid-producing microalgal strains suitable for these special conditions are ideal. Using native species can be a superior option over non-natives in an environmental sense to prevent spread of exotics or genetically modified organisms. There are reports of alkaliphilic microalgae from extreme environments, including soda lakes; however, limited efforts have isolated and characterized algae meeting these criteria from freshwater sources, especially from Florida, USA (Selvarajan et al. 2015). The purpose of this study was to exploit the local, lipid-producing, alkali-tolerant / alkaliphilic biodiversity of microalgae, and fill this genetic resource gap. A starting point was to investigate the alkaline tolerant species of our local Lake Okeechobee, Florida.
Lake Okeechobee (26°56′ N, 80°48′ W), located in subtropical south-central Florida, is the largest lake in the southeastern United States. It is a relatively shallow eutrophic lake with a mean depth of 2.7 m (Davis and Marshall 1975, Canfield and Hoyer 1988, James et al. 1995). The lake water levels may undergo wide diel and annual pH fluctuations through man made water control structures and through photosynthesizing phytoplankton (Hagerthey et al. 2011). A high pH in the lake water column can result from photosynthetic withdrawal of carbon dioxide from the water shifting the carbon dioxide-carbonic acid-carbonate equilibrium or the natural limestone benthos. Therefore, we hypothesized that there is a strong possibility for occurrence of native alkali-tolerant microalgae in Lake Okeechobee outflow waters.
In this study, our goal was to isolate and characterize high biomass and lipid producing alkali-tolerant or alkaliphilic microalgae from Lake Okeechobee as novel biological resources for the production of algae-based products. The use of strains with alkaliphilic growth requirements would alleviate the problem of contamination during mass production.
MATERIALS AND METHODS
Sample collection and enrichment cultures
Surface water grab samples were obtained from different Lake Okeechobee outflows and canals along the Everglades Agricultural Area (EAA) in July 2013. The map of the southern portion of Lake Okeechobee and the 12 sampling sites used in the study (darkened circles) is shown in Supplementary Fig. S1. Water samples were stored in a cooler until transferred to the lab for processing.
Zarrouk’s medium (Vonshak 1993) with an initial pH of 9 and 10 was used for enrichment. Initial enrichment culture flasks for each sampling site consisted of equal volume of 1 : 1 (v/v) Zarrouk’s medium and water sample. There were three replicate flasks for each sampling site. The flasks were incubated on a rotary shaker at 150 rpm and 25°C under continuous illumination of 60 μmol photons m−2 s−1. After 35 days of cultivation, culture flasks showing visible algal growth were transferred into fresh, pure Zarrouk’s medium supplemented with Gamborg’s B5 solution (1,000×; 1 mL L−1) and 30 g L−1 of NaSiO3.
Selective enrichment of alkali-tolerant isolates
To select for alkali-tolerant isolates, the enrichment cultures were incubated in Erlenmeyer flasks containing modified Zarrouk’s medium at different initial pH values of 9, 10, 11, and 12. The pH of the medium was adjusted using a 10 mol−1 KOH solution. Three replicate flasks were prepared for each pH treatment. Algal suspension from initial enrichment culture was used to inoculate each flask to an initial optical density (OD) of 0.2 at 600 nm. Cultures were incubated on a rotary shaker at 150 rpm and 25°C under a constant light intensity of 60 μmol photons m−2 s−1 for 35 days.
Growth was assessed using chlorophyll a as an abundance indicator with quantifications carried out every 7 days. A 5 mL aliquot from each Erlenmeyer flask was collected and filtered using a Whatman GF/A (1.6 μm) glass microfiber filter (Whatman Ltd., Buckinghamshire, UK). Sample processing for chlorophyll extraction was adapted from Dere et al. (1998). For this, each filter containing biomass was placed in a 15 mL conical Falcon tube, and 5 mL of 100% acetone was added. Tubes were covered with aluminum foil and stored at 4°C for 24 h. Samples were then centrifuged at 5,000 rpm for 10 min. The supernatant was transferred into a 2 mL cuvette and absorbance was measured at 662 and 646 nm for chlorophyll a and b, respectively using a Thermo Scientific Spectronic 200 photospectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Pigment concentration was calculated according to the formulas of Lichtenthaler and Wellburn (1983).
Isolation and identification of alkali-tolerant strains
Zarrouk’s agar medium adjusted to an initial pH of 9 and 10 were used to isolate alkali-tolerant algae. To obtain pure cultures of individual algal isolates, a 1 mL aliquot of the enrichment culture from each of the pH 9 and 10 treatment flasks was plated on corresponding Zarrouk’s agar (15 g L−1) plates. The plates were incubated for three weeks at 25°C under continuous light (40 μmol photons m−2 s−1). Individual algal colonies from the alkaline selective agar plates were then streaked onto another plate containing agar medium amended with azithromycin, kanamycin, and streptomycin (10 μg mL−1 each) to render isolates axenic. Clonal isolates were then transferred to fresh Zarrouk’s medium having a corresponding initial pH and maintained at 25°C.
Genus-level taxonomic identification was based on morphology and carried out using algae identification guides (Lange-Bertalot 2001, Bellinger and Sigee 2015, Wehr et al. 2015). Microscopy of algal isolates was carried out on an Olympus BX51 microscope with attached camera (Olympus DP70) using the corresponding DP controller and manager software applications (Olympus Optical Co. Ltd., Hamburg, Germany). Identification of more complex taxa was carried out using scanning electron microscopy (SEM) detailed below.
Scanning electron microscopy
Since Fistulifera sp. 154-3 was selected for further study, a more detailed morphological analysis of this strain was performed using SEM (JSM-5900L V; JEOL, Tokyo, Japan). The samples for SEM were prepared using the modified methods of hot hydrogen peroxide (H2O2) (Taylor et al. 2007). For this, 10 mL of culture was pipetted into a 15 mL Falcon tube and centrifuged at 3,000 ×g for 15 min at 25°C and the supernatant was removed. The cells were centrifuged and washed twice with 15 mL of sterile BG11 medium. The washed cells were resuspended in 1 mL of deionized water, transferred into a glass beaker and then 10 mL of hydrogen peroxide (H2O2) was added (30–35%). The pellet suspended in H2O2 was heated to 90°C in a hot water bath for four hours. Once the solution appeared clear, three drops of 50% hydrogen chloride (HCl) were added and the solution was then transferred into a glass centrifuge tube and washed with sterile deionized H2O three times. Two hundred microliters of washed biomass was pipetted onto a glass slide and allowed to dry completely. The slide was fixed onto an aluminum stub and gold coated (sputter coater) for SEM imaging.
Alternatively, the samples were prepared using critical point drying (Samdri-PVT-3D; Tousimis, Rockland, MD, USA). Fifty mililiters of culture was pipetted into a 50 mL glass centrifuge tube and centrifuged at 300 ×g for 10 min. The supernatant was removed and replaced with 50 mL of sterile BG11 to remove medium salts and prevent cell lysis. Next, the samples were centrifuged at 300 ×g for 10 min, the supernatant removed, and 20 mL of 2% paraformaldehyde in 0.1 M phosphate buffer was added and incubated at room temperature for 2 h. After incubation, samples were centrifuged at 300 ×g for 10 min and the fixative was removed. Algae cells were then washed with 20 mL of sterile deionized H2O and cells were allowed to settle. Once settled, 1 mL of cells was pipetted onto a 0.2 μm filter (25 mm; Gelman, Ann Arbor, MI, USA), filtered on low, and then immediately transferred into 30% ethyl alcohol (EtOH). Filters were then transferred sequentially into 50, 70, 90, and 100% ethyl alcohol, allowing 10 min incubation time for each dehydration step. Filters were placed in the critical point dryer (34°C, 1,350 psi) and then gold-coated for SEM imaging.
Molecular phylogenetic analyses
A 1 mL aliquot of axenic exponential phase cells of Fistulifera sp. 154-3 was transferred into a sterile Eppendorf tube and centrifuged. DNA was extracted using a DNeasy PowerPlant Pro kit (Qiagen, Carlsbad, CA, USA). The nuclear-encoded, small ribosomal subunit (18S rRNA) gene was amplified with polymerase chain reaction (PCR) using GoTaq Green Master Mix (Promega, Madison, WI, USA) with ss3 and ss5 primers (Rowan and Powers 1991, Matsumoto et al. 2014). To fortify the sequence annotation, internal primers were designed and used including the forward primer fistF: 5′-GGTCCTATTTTGTTGGTTTGCG-3′ and the reverse primer fistR: 5′-CGCAAACCAACAAAATAGGACC-3′. The PCR reaction was carried out on an Eppendorf Master Cycler (Nexus GX2; Eppendorf, Hamburg, Germany) using the conditions of 95°C for 45 s, then 35 cycles at 50°C for 45 s, and extension with 72°C for 2 min. The PCR product was visualized on an agarose gel (1%) and then Sanger sequenced by Eurofins Genomics (Louisville, KY, USA). The gene sequence for Fistulifera sp. 154-3 is deposited in GenBank (National Center for Biotechnology Information, NCBI) under the accession number KY982278.
BLAST (Basic Local Alignment Search Tool, NCBI) was used for locating strains similar to the 18S rRNA of Fistulifera sp. 154-3. Thirty-four diatom sequences were obtained and aligned using MUSCLE (SeaView v4.4) (Edgar 2004), and then manually annotated based on conserved regions. A total of 1,698 bps of the 18S rRNA gene were used from a total of 34 taxa including Navicula lanceolata (AY485484) and Navicula reinhardtii (AM501976) as the outgroup. Phylogenetic trees were constructed using Bayesian inferences (BI) and maximum likelihood (ML) with MrBayes (v3.3.6) and RAxML (7.2.7), through the CIPRES network (v.3.1) (Guindon and Gascuel 2003, Miller et al. 2010). The ML analysis was carried out using the GTR + I + G model, based on jModelTest, assuming heterogeneous substitution rates and gamma substitution of variable sites (proportion of invariable sites [pINV] = 0.390, shape parameter [α] = 0.420, number of rate categories = 4); bootstrap resampling on 1,000 replicates. The BI analysis was conducted using MrBayes 3.6 (Ronquist and Huelsenbeck 2003). Four Metropolis-coupled Markov chain Monte Carlo (one cold and three heated) were run for 5 × 106 generations and the first 25% were discarded as burn-in and the following data sets were sampled with a frequency of every 100 generations.
Screening of strains for lipid accumulation
To determine which of the three isolates accumulated large quantities of lipids, Nile red fluorescence dye (ACROS Organics, Morris Plains, NJ, USA) was used to stain algal cells, both for visualizing lipid droplets within algal cells and for estimating lipid content (Chen et al. 2009). Lipid visualization and quantification were performed by staining algae cells with Nile red and diluting 1 mL of culture to 0.1 OD600 and adding dimethyl sulfoxide (20%) and Nile red (1.5 μg mL−1) and incubating in the dark for 15 min. Samples were screened in 96-well plates, for autofluorescence and lipid fluorescence using a plate reader (Synergy HTX; Bio Tek, Winooski, VT, USA) and corresponding Gen 5 Analysis software (v3.0) at excitation and emission wavelengths of 530 and 575 nm, respectively. Fluorescence responses of algae were then translated into lipid concentrations using a Triolein lipid standard curve.
Effect of pH on biomass and lipid accumulation of isolated algae
Once obtained in axenic culture, Fistulifera sp. 154-3 was inoculated into Zarrouk’s medium, while Chloroidium sp. 154-1 and Chlorella sp. 154-2, which did not grow in Zarrouk’s medium, were inoculated into BG11 medium supplemented with 16.8 g L−1 of NaHCO3. Biomass and lipid productivity of Chloroidium sp. 154-1, Chlorella sp. 154-2, and Fistulifera sp. 154-3 were evaluated at pH 7–11 (increments of 0.5). Individual algal species were grown in 150 mL flasks with four replicates each. Aliquots of 1 mL were taken every 5th day for 20 days for both biomass and lipid measurements. Biomass productivity was assessed by filtering 1 mL of culture onto pre-weighted glass microfiber filters (1.2 μm, Whatman GF/C). Filters were dried to constant weight in an oven at 60°C. Lipid content was assessed using the Nile red method and converted lipid value using a Triolein lipid standard curve. Lipid productivity was calculated by multiplying biomass productivity and the lipid content of algae as suggested by Griffiths and Harrison (2009).
Growth rate, lipid gravimetric, and compositional analyses
The strain that demonstrated highest lipid productivity (Fistulifera sp. 154-3) was selected for specific growth rate and doubling time quantification by inoculating the alga in pH 10 modified Zarrouk’s medium. Biomass, OD (440 nm), and Nile red lipid quantifications were carried out every two days for 23 days, as previously described.
To compare the Nile red method with gravimetric lipid analyses and determine the fatty acid composition of algal biomass, our diatom strain was cultured in triplicate 3 L flasks and harvested on day 10 (exponential phase) by centrifugation at 2,000 ×g (GH 3.7; Beckman Coulter, Indianapolis, IN, USA) and lyophilized. For lipid extraction, 1 g of dry algal biomass was placed in glass centrifuge tubes and a modified method by Axelsson and Gentili (2014) was used to extract total lipids in triplicate. Total lipid extract was analyzed to characterize the fatty acid methyl esters (FAMEs). FAMEs analyses were conducted at Gorge Analytical (Gorge Analytical, LLC., Hood River, OR, USA) according to the AOCS Official Method Ce 1–62 (3) (American Oils Chemists Society 2005). Briefly, FAMEs were prepared by transesterification using methanolic hydrochloric acid with an internal standard (Tripentadecanoin; Sigma-Aldrich, St. Louis, MO, USA) and then analyzed with a standard mixture of C8–C24 FAMEs (Sigma-Aldrich) using gas chromatography with a flame ionization detector (Agilent Technologies, Santa Clara, CA, USA).
Statistical analysis
For the selective enrichment of alkali-tolerant isolates, a total of two samples were used with three replicates each at different pH 9–12 and the significant differences between samples at varying pH were determined using a repeated measure, one-way analysis of variance (ANOVA) (Stemmler et al. 2016). Significant differences in biomass and lipid accumulation between the different isolated algae with four replicates were also determined using a one-way ANOVA. A one-way ANOVA was used to determine the possible effect of varying pH 7–12 on biomass and lipid accumulation of Fistulifera sp. 154-3. A one-way ANOVA was also used to determine significant differences between the mean gravimetric lipid weight and the lipid concentration determined using Nile red. All significant differences between treatment means for each variable were compared using a Tukey post-hoc analysis at p < 0.05. All statistical analyses were performed using SPSS software package ver. 22.0 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS AND DISCUSSION
Initial enrichment and isolation
The primary goal of this work was to isolate alkali-tolerant or alkaliphilic algal strains with lipid accumulating properties that could potentially be used for production of biofuel and / or nutraceutical compounds. Using algal strains with extremophile properties for mass cultivation can circumvent contamination in both outdoor and indoor cultivation schemes (Chi et al. 2011). In this work, we used surface water samples from canals coming directly from Lake Okeechobee (FL), whose pH ranges from slightly alkaline to strongly alkaline depending on spatiotemporal factors (Canfield and Hoyer 1988, James et al. 1995). This characteristic of lake water led us to hypothesize that alkali-tolerant strains of algae could be isolated from these samples.
A total of 12 surface water samples were collected from different outflow sites located near Lake Okeechobee as potential sources of alkali-tolerant microalgae. Presumptive alkali-tolerant microalgae were isolated using an alkaline selective enrichment approach. Out of those 12 samples, only site S-308 showed visible growth in two of the replicate cultures (flasks A and B) at pH 9 and 10, respectively (Supplementary Fig. S2). Further selection of cultures from the initial enrichment flasks obtained by using full strength Zarrouk’s medium is shown in Supplementary Fig. S2. In this selective enrichment experiment, only cultures with an initial pH of 9 and 10 had an increase in chlorophyll a content until the end of the cultivation (day 35). Growth of the enrichment cultures from culture flasks A was significantly higher in pH 9 and 10 in comparison to both cultures of flasks A and B inoculated in pH 11 and 12 (p < 0.001, α = 0.05). Enrichment cultures from flasks A were significantly higher in chlorophyll a content than the culture flasks B inoculated in both pH 9 and 10 (p < 0.001, α = 0.05). There was no significant difference in chlorophyll a content between the culture flasks A inoculated at both pH 9 and 10 (p = 0.996, α = 0.05). Chlorophyll content in sample flasks A inoculated in pH 9 and 10 were significantly and consistently higher throughout the 35-day incubation period than flasks B in pH 9, 10, and 12, and as a result, was used for subsequent isolation of algae. In contrast, the cultures with initial pH of 11 and 12 showed some growth initially but died on week 3 and were voided for algal isolations.
Three alkali-tolerant / alkaliphilic strains of microalgae were isolated from the enrichment cultures. Those strains were identified based on morphological features as the chlorophytes Chloroidium (strain 154-1) and Chlorella (strain 154-2), and a pennate diatom Fistulifera (strain 154-3). Chloroidium sp. 154-1 and Chlorella sp. 154-2 were isolated from Zarrouk’s agar pH 9 plates, while Fistulifera sp. 154-3 was isolated from a Zarrouk’s agar pH 10 plate. When stained with Nile red, a stain often used for lipid quantification (Han et al. 2011), Chloroidium sp. and Chlorella sp. did not show significant accumulation of lipids, while Fistulifera sp. accumulated considerable amounts of lipids within the cells (Fig. 1). Thus, Fistulifera sp. 154-3 was chosen for lipid analysis.
Biomass and lipid accumulation of algal isolates under increasing pH
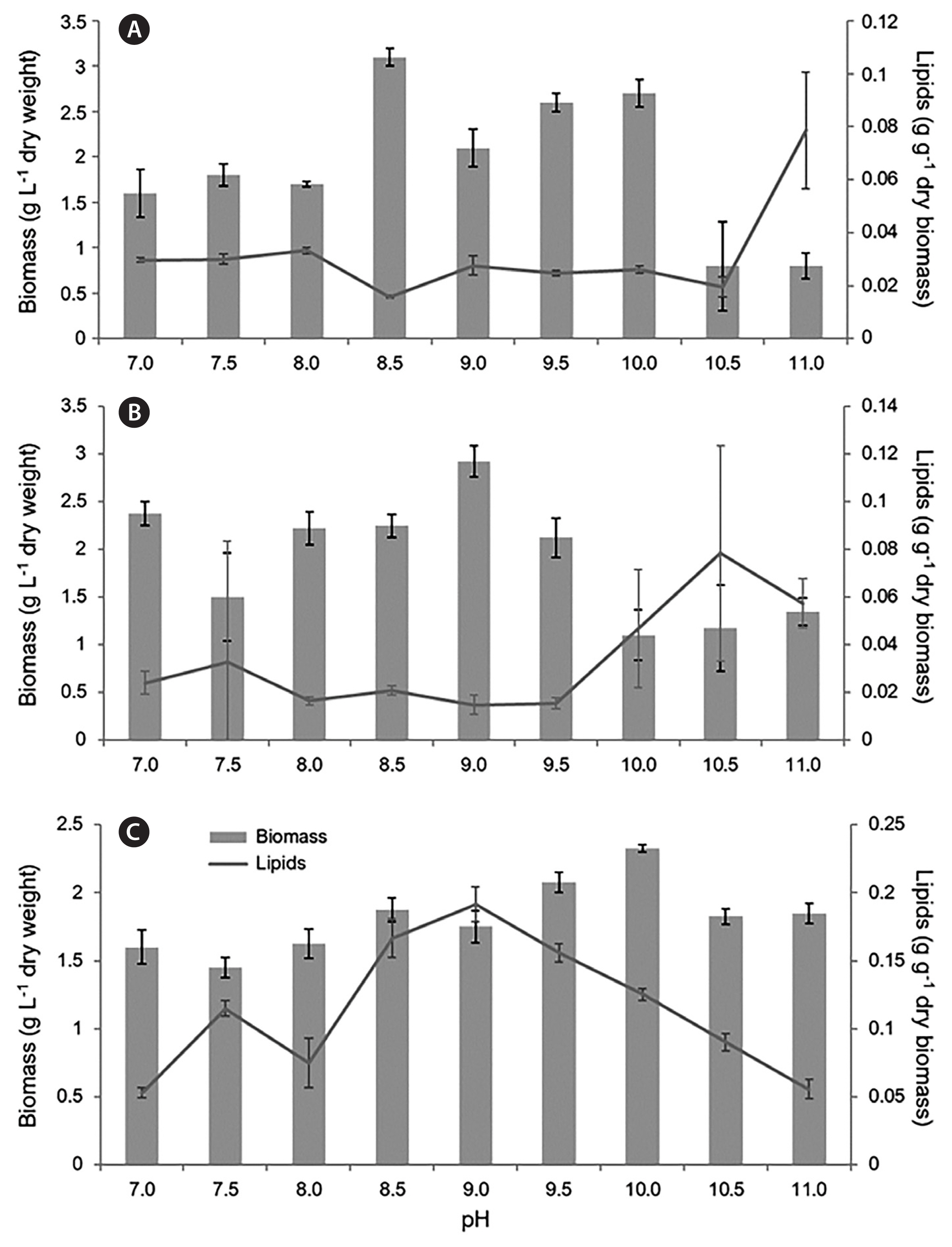
Biomass production and lipid accumulation of the isolated microalgae varied depending on the strain and pH of the medium (Supplementary Table S1). Over the 20-day cultivation period, Chloroidium sp. 154-1 had the highest mean biomass (2.64 g L−1) at pH 8.5. The highest mean lipid content for Chloroidium sp. 154-1 occurred at pH 9, with up to 4.51% dry biomass. The highest mean biomass and lipid content for Chlorella sp. 154-2 was observed in pH 9 with 2.95 g L−1, and pH 10.5 with 5.86% dry biomass. The highest mean biomass and lipid content for Fistulifera sp. 154-3 was observed at pH 10 with 2.68 g L−1, and pH 9 with up to 8.78% dry biomass over the 20-day incubation period.
During the 20-day sampling period, the highest lipid accumulation among algal strains tested was recorded for Fistulifera sp. 154-3 on day 10. The biomass and lipid percentage data from only day 10 are therefore presented for all three algal strains, the data from the rest of the sampling days (5, 15, and 20) are provided in Supplementary Table S1. The dry biomass yield and lipid content for all three strains, Chloroidium sp. 154-1, Chlorella 154-2, and Fistulifera sp. 154-3 on the 10th day of cultivation, are shown in Fig. 2. A maximum dry biomass of 3.05 g L−1 was recorded for Chloroidium sp. 154-1 and 2.93 g L−1 for Chlorella sp. 154-2 and the lipid content in those two strains was never >11% across the pH levels tested over 20 days of cultivation. The highest dry biomass (3.1 g L−1) of Chloroidium sp. 154-1 was observed in pH 8.5, while the highest lipid accumulation (9.34%) was found at pH 11 (Fig. 2A). In Chlorella sp. 154-2, the highest dry biomass of 2.93 g L−1 occurred at pH 9, while the highest lipid accumulation (3.67%) resulted in the pH 10.5 treatment (Fig. 2B). The mean values for lipid content of Chloroidium sp. 154-1 at pH 11 and Chlorella sp. 154-2 at pH 10.5 were highly variable, as most of the replicates ceased to grow and flasks contained mostly dead cell debris (Supplementary Fig. S3). In Fistulifera sp. 154-3, maximal lipid accumulations of 16, 17, and 19% were recorded at pH 9.5, 8.5, and 9, respectively.
Chloroidium sp. 154-1 had the highest lipid concentration on day 5 of incubation with up to 11.03% of dry biomass. The lipid content gradually decreased for the remaining incubation period. The highest mean lipid content occurred in pH 9.5 with up to 4.51% of dry biomass. Using pH 8.0 as a control, the biomass of Chloroidium sp. 154-1 was significantly lower than biomass found at pH 8.5, 9.5, 10, and 10.5 (p = 0.001, p = 0.014, p = 0.003, p < 0.001, α = 0.05). The biomass at pH 8.5 was consistently higher than all other pH levels on days 10, 15, and 20. The biomass of Chloroidium sp. 154-1 was significantly higher at pH 8.5 than 7, 7.5, 8, and 11 (p = 0.005, p = 0.011, p = 0.001, p < 0.001, α = 0.05) on day 10.
For Chlorella sp. 154-2, using pH 8 as a control on day 10 of cultivation, the only significantly higher biomass occurred at pH 10 (p = 0.041, α = 0.05). The biomass accumulation of Chlorella sp. 154-2 in pH 9 was significantly higher than the pH levels of 7.5, 10, 10.5, and 11 (p = 0.004, p = 0.000, p = 0.000, p = 0.001, α = 0.05). Conversely, the lipid content at pH 10.5 was significantly higher than the lipid content observed at pH 7, 8, 8.5, 9, and 9.5 (p = 0.004, p = 0.001, p = 0.003, p = 0.001, p = 0.001, α = 0.05).
On day 10 of incubation, the biomass of Fistulifera sp. 154-3 was significantly lower in pH 8 than in pH 9.5 and 10 (p = 0.007, p = 0.000, α = 0.05). The biomass of Fistulifera sp. 154-3 at pH 10 was significantly higher than pH levels of 7, 7.5, 8, 8.5, 9, 10.5, and 11 (p < 0.007, α = 0.05). There were no significant differences between the biomass of Fistulifera sp. 154-3 at pH 9.5 and 10 (p = 0.366, α = 0.05). The lipid content of Fistulifera sp. 154-3 found in pH 8 was significantly lower than that observed in pH 8.5, 9, 9.5, and 10 (p = 0.000, p = 0.000, p = 0.000, p = 0.011, α = 0.05). The lipid content within Fistulifera sp. 154-3 was significantly higher at pH 9 than in pH 7, 7.5, 8, 10.5, and 11 (p < 0.001, α = 0.05). Fistulifera sp. 154-3 cultured between pH 8.5 and 9.5 showed 3 to 5 times higher lipid content than Chloroidium sp. 154-1 and Chlorella 154-2 (Fig. 2C).
When comparing the biomass and lipid content of the three isolates on day 10 of cultivation, the biomass of Chloroidium sp. 154-1 and Chlorella sp. 154-2 were both consistently higher than Fistulifera sp. 154-3 in all pH levels tested except 11. Conversely, the lipid content of Fistulifera sp. 154-3 was higher than both Chloroidium sp. 154-1 and Chlorella sp. 154-2 in all pH levels tested. Since Fistulifera sp. 154-3 displayed higher concentrations of lipids, this strain was chosen for further productivity calculations and fatty acid determinations.
The biomass and lipid productivity of Fistulifera sp. 154-3 were evaluated at pH 7–11 in increments of 0.5, in Zarrouk’s medium. The strain was able to grow over a wide range of pH (Supplementary Fig. S4A); the highest biomass productivity (0.415 g L−1 d−1) was recorded after five days of cultivation at pH 8.5. This biomass productivity on day 5 was almost 2-fold significantly higher than at pH 7, 9, 9.5, 10.5, and 11 (p = 0.048, α = 0.05). Thereafter, the highest biomass productivity was recorded at pH 10 on day 10, 15, and 20 of cultivation with resulting productivities of 0.233, 0.240, and 0.169 g L−1 d−1, respectively (Supplementary Fig. S4A). The biomass productivities at pH 10 on day 10 and day 15 were significantly higher than all the rest of the pH treatments (p = 0.007, α = 0.05). The difference in medium pH values resulted in substantial variation in the lipid productivity throughout cultivation (Supplementary Fig. S4B). The highest mean lipid productivity among the pH treatments was found on day 10 of cultivation. On day 10 of cultivation, the lipid productivities of Fistulifera sp. 154-3 ranged between 8.08 and 39.95 mg L−1 d−1. Fistulifera sp. 154-3 cells showed higher lipid productivity at pH 8.5, 9, 9.5, and 10, than at pH 7, 7.5, 10.5, and 11. After quantification of lipid and biomass productivity, specific growth and doubling time of the diatom were investigated.
A separate growth run demonstrated that the average specific growth rate of Fistulifera sp. 154-3 was μ = 0.832 d−1 with a corresponding doubling time of 20 h. The specific growth rate and doubling time is comparable to most diatoms previously described (Griffiths and Harrison 2009). Additionally, when inoculated in increasing pH from 7 to 11, all of the initial pH levels were naturally altered until reaching pH of 10.2–10.3 after a week of incubation and remained at that level thereafter (Supplementary Fig. S5). The pH changed drastically after one day of incubation for all treatments except that of pH 10, which remained constant. As a result, Fistulifera sp. 154-3 is a diatom capable of manipulating the medium pH through photosynthesis to suitable alkalinity levels for proper growth. A naturally alkalizing diatom can be an ideal tool in mass cultivation where a constant pH is kept without artificial buffers.
From Lake Okeechobee, we have isolated two algal strains that could be characterized as alkali-tolerant and one alkaliphilic. The S-308 site water sample from which these strains were isolated had a pH of 8.2 (± 0.2). Chloroidium sp. 154-1 and Chlorella sp. 154-2 (Fig. 2) with optimal growth ≥ pH 8–9 were designated as biomass-accumulating alkali-tolerant algae, according to Jones et al. (1994) and Gimmler and Degenhard (2001). The diatom Fistulifera sp. 154-3 (Figs 1, 2, Supplementary Figs S4 & S5) with an optimal growth from pH 8.5 up to 10.3, and highest lipid productivity at pH 10, is designated as alkaliphilic (Kroll 1990, Gimmler and Degenhard 2001). Since Fistulifera sp. 154-3 demonstrated high alkaline tolerance and moderate biomass and lipid productivities, it was chosen for further characterizations.
SEM confirms genus Fistulifera
SEM microphotographs of Fistulifera are depicted in Supplementary Fig. S6. The individual cells of Fistulifera sp. 154-3 are below 10 μm in size (Fig. 1). Frustules of Fistulifera sp. 154-3 have symmetrical valves up to 7 μm in length; substituting valve length for raphe length since the genus is lightly silicified resulting in a reduced valve margin. The genus Fistulifera is characterized by light silicification of the frustule, a unique raised raphe sternum, and fistula opening in the central region of the valve (Supplementary Fig. S6E–G). A few species have been characterized within this genus including Fistulifera solaris S. Mayama, M. Matsumoto, K. Nemoto & T. Tanaka, F. saprophila (Lange-Bertalot & Bonik) Lange-Bertalot, F. iranensis (Hustedt) Lange-Bertalot, and F. pelliculosa (Kützing) Lange-Bertalot (Zgrundo et al. 2013, Matsumoto et al. 2014, Guiry and Guiry 2018). Fistulifera saprophila is found in eutrophic waters, while F. pelliculosa is restricted to oligotrophic waters. Fistulifera pelliculosa and F. saprophila both secrete mucilage when cells cease to replicate (Lewin 1955, Zgrundo et al. 2013). Fistulifera solaris differs since it is a marine species and is slightly larger (Matsumoto et al. 2014). Fistulifera sp. 154-3 can be distinguished from the other previously described Fistulifera species by its lack of mucilage production throughout its growth cycle and has a thinner girdle band (Supplementary Fig. S6B). Interestingly, Fistulifera sp. 154-3 also forms colonies of 2–10 cells, as documented for F. saprophila (Zgrundo et al. 2013).
The ubiquitous genus Fistulifera is comprised of species that occur in fresh, brackish or saline waters, at varying degrees of pollution, pH, and salt concentration. To date, the species that are well characterized within this genus include: Fistulifera solaris, F. iranensis, F. saprophila, and F. pelliculosa. Studies on this genus have focused on the strain F. solaris JPCC DA0580, which has high lipid accumulation (40–60%), but limited to growth in artificial seawater (Matsumoto et al. 2010, Sato et al. 2014, Muto et al. 2015, Tanaka et al. 2015) with possible growth in brackish water (Satoh et al. 2013).
Conversely, Fistulifera sp. 154-3 thrives in modified Zarrouk’s medium, adjusted at pH 10 and 1.4% salinity (Supplementary Fig. S3C). Fistulifera sp. 154-3 accumulates the highest levels of lipids on day 10 at pH 9 and when biomass is taken into account, lipid productivities at pH 8.5–10 are superior to the other treatments (Supplementary Fig. S4A & B). Additionally, the diatom is capable of naturally manipulating the media to suitable pH levels near 10.2 (Supplementary Fig. S5). Fistulifera sp. 154-3 demonstrates how adaptation of this genus to varying physicochemical conditions, from oligotrophic, eutrophic, to increasing halo-alkaline conditions, could be conducive to minimizing invading organisms during cultivation. Thus, we identified Fistulifera sp. 154-3 as an alkaliphilic alga that could facilitate the mass cultivation of algae in both indoors and outdoors schemes.
In using Fistulifera sp. 154-3 for outdoor cultivation, sodium bicarbonate becomes an ideal component in cultivation. Sodium bicarbonate is a superior carbon source for algal cultivation since the natural salt concentration increases the pH of the medium. Atmospheric carbon can also be captured in the form of bicarbonate at a fraction of the cost, and this carbon can be stored and transported without significant loss to the atmosphere. Additionally, using bicarbonate and a high pH cultivation environment leads to minimal carbon loss by outgassing (Chi et al. 2011). As Fistulifera sp. 154-3 uses up the bicarbonate in solution, the medium becomes a carbon sink and pulls CO2 from the air to maintain the bicarbonate/ carbonate buffer system and provide the diatom with a carbon source. Using sodium bicarbonate to increase pH values of the culture medium can be a good strategy, not only in preventing culture contamination, but also as a factor stimulating lipid accumulation (Gardner et al. 2011).
Molecular phylogenetic analyses
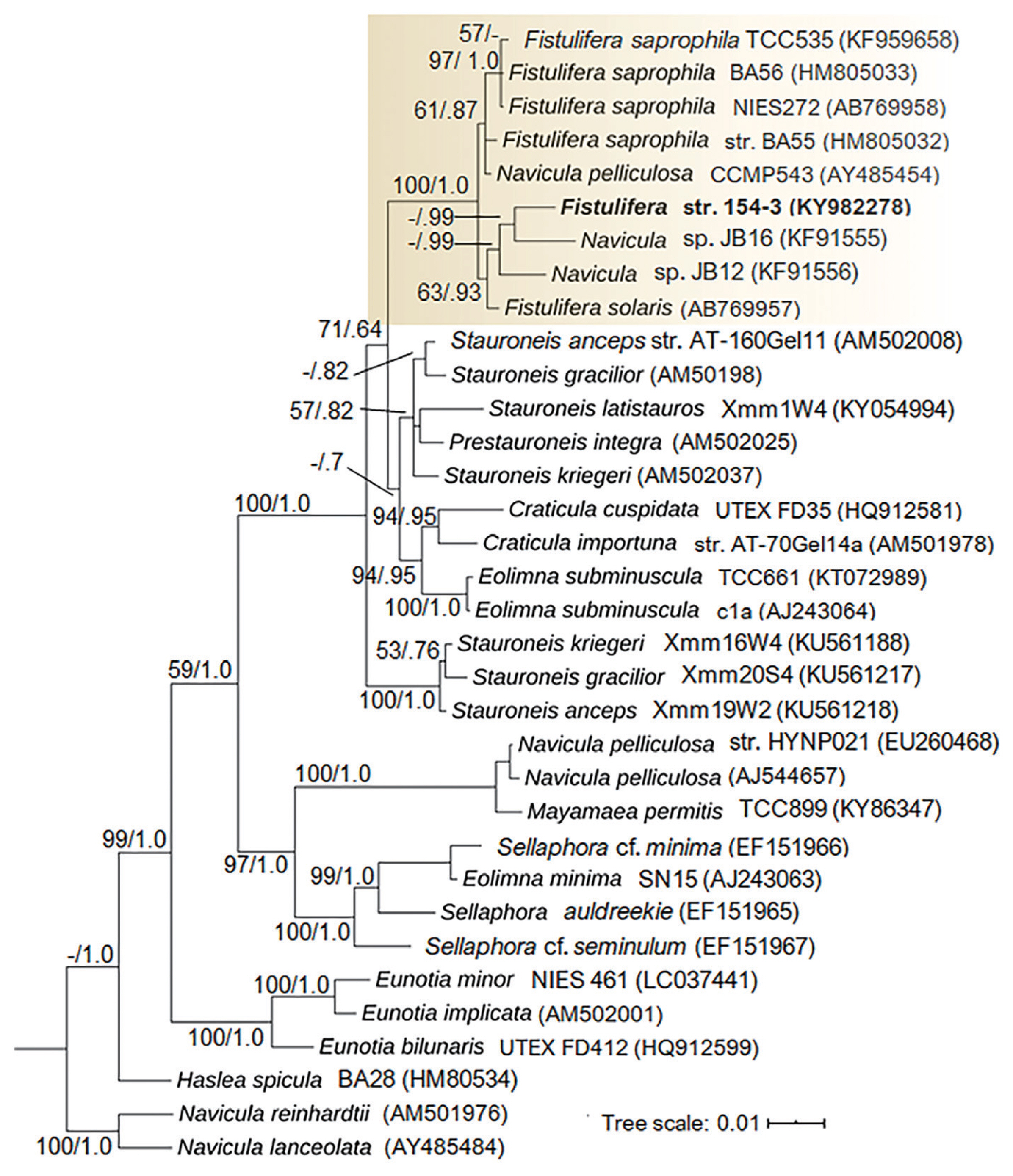
A more detailed taxonomic identification of Fistulifera sp. 154-3 was performed using the 18S rRNA gene (Fig. 3). The phylogenetic analysis shows that the genus Fistulifera is a well-supported (bootstrap [BS], 100%; posterior probability [PB], 1.0) monophyletic clade (box in Fig. 3). In the analysis, several sequences of Fistulifera pelliculosa including AJ544657 and EU260468 did not fall within the Fistulifera clade, but with Mayamaea sp. (KY863471). However, the isolate Fistulifera sp. 154-3 formed a well-supported distinct subclade that included Navicula sp. JB12 (KF791556) and JB16 (KF791555) (BS, 63%; PB, 0.93). The subclade that contains Fistulifera sp. 154-3 and the Navicula sp. strains are isolated from high alkaline waters and soils, respectively, whereas the remaining Fistulifera strains are located in separate subclades with related saline and freshwater strains; which delimited into strongly supported branches for F. saprophila (BS, 97%; PB, 1.0) and F. pelliculosa (BS, 61%; PB, 0.87) as well. The phylogenetic distance of Fistulifera sp. 154-3 suggests that it might represent a novel species to science, but a more detailed characterization of this strain is necessary for a proper taxonomic description.
Lipid analyses
To compare the Nile red method with traditional gravimetric techniques, Fistulifera sp. 154-3 was cultured until the exponential phase and analyzed in triplicate. The mean lipid content of the diatom was 31.0 and 23.2% for the Nile red and gravimetric methods, respectively (Supplementary Fig. S7). There was no significant difference in the mean lipid content determined with either detection method (p = 0.277, α = 0.05).
Lipid analyses of Fistulifera sp. 154-3 revealed a profile predominantly abundant in monounsaturated followed by saturated and then polyunsaturated fatty acids (Table 1). Monounsaturated carbon methyl chains include 52% PA (C16:1c), 1.3% myristoleic acid (C14:1c), 5% co-elution of oleic / elaidic acid (C18:1c; C18:1t), and 0.74% cis-10-heptadecenoic acid (C17:1). In terms of saturated compounds, Fistulifera sp. 154-3 can produce around 17% palmitic acid (C16:0), 5% myristic acid (C14:0), and 0.41% stearic acid (C18:0). Polyunsaturated compounds include about 16% cis-5,8,11,14,17 eicosapentaenoic acid (C20:5n3), 1.2% co-elution of linoleic / linolelaidic acid (C18:2c; C18:2t), 0.34% arachidonic acid (C20:4n6), and 0.31% of γ-linolenic acid (C18:3n6).
Most of the fatty acids accumulated by our Fistulifera sp. 154-3 are represented by PA (C16:1c), palmitic acid (C16:0), and Cis-5,8,11,14,17 eicosapentaenoic acid (C20:5n3) (Table 1). The lipid profile of Fistulifera sp. 154-3 has implications for both medicinal and environmental sectors. This diatom produces fatty acids ideal for fuel substitutes as it produces many long, saturated carbon chains, such as palmitic acid, and polyunsaturated carbon compounds. Additionally, PA has an industrial value for polyethylene production and biofuels (Nguyen et al. 2015).
An additional key potential commercial application of Fistulifera sp. 154-3 lies in the production of omega fatty acids as a human supplement, including PA (ω-7) and eicosapentaenoic acid (ω-3). Omega-7 fatty acids can be extracted from Fistulifera sp. 154-3 without the risk of fish-associated toxic contaminants and avoid competition with the traditional agricultural sources of these omega fatty acids including macadamia nuts (Macadamia integrifolia) and sea buckthorn berries (Hippophae rhamnoides) (Bal et al. 2011, Copat et al. 2012). To date, PA can be sourced from a variety of organisms including yeasts and algae, but restricted to the type of cultivation used. Yeast-based sources have shown to produce only up to 16% palmitoleic acids and limited to fermentative schemes (Kolouchová et al. 2015).
Alternatively, the marine alga Nannochloropsis salina Hibberd can accumulate PA between 22 to 30% of its total fatty acids (Ma et al. 2016) with possible increases of up to 35% based on altering cultivation light and temperature regimes (Van Wagenen et al. 2012). Another alga, Heterococcus sp., was found to produce 20–55% PA in temperatures of 4–22°C, but is only suitable for cultivation in cold temperate climates (Nelson et al. 2013). In the present study, the alkaliphilic diatom Fistulifera sp. 154-3 was able to produce up to 52% PA when exposed to a warm temperature of 25°C, allowing for cultivation in a subtropical climate.
Our isolate Fistulifera sp. 154-3 accumulates around (20–30%) lipids, with a lipid productivity of around 40 mg L−1 d−1, and we believe that by manipulating cultivation regimes, a higher lipid production can be achieved (D’Ippolito et al. 2015). In the future, we aim to optimize the biomass and lipid productivity of Fistulifera sp. 154-3 by manipulating the nutrient composition (bicarbonate and B vitamins), salt concentration, temperature, and light intensity during cultivation. Additional morphological and molecular analyses are required to reveal the true phylogenetic placement of our Fistulifera isolate.
CONCLUSION
High halo-alkaline conditions in algae cultivation may restrict the persistence of contaminants and competing organisms, which can facilitate the scaling-up process. Since there is limited number of extremophilic algae available for cultivation, the purpose of this study was to explore Florida native, lipid-producing, alkali-tolerant / alkaliphilic microalgae diversity. Bioprospection and initial selection for extremophilic algae from Lake Okeechobee resulted in the isolation of three algal strains: two alkali-tolerant isolates and one alkaliphilic isolate. Using Nile red to determine intracellular lipid concentrations revealed an alkaliphilic diatom with high lipid productivity around 40 mg L−1 d−1. Morphology and 18S rRNA gene sequence phylogeny showed the diatom belonged to a genus of small raphid diatoms, Fistulifera. Gravimetric analyses indicated a diatom where 30% of its biomass are lipids, and gas chromatography showed a fatty acid profile to mainly consist of palmitoleic, palmitic, and eicosatetraenoic fatty acids. In conclusion, the isolated strain Fistulifera sp. 154-3 is an alkaliphilic diatom with potential use in mass cultivation systems to produce high-value lipid compounds, such as ω-7, ω-3, and palmitic acid. Omega-7 and ω-3 can be used for nutraceutical production, and palmitic acid can be used as a feedstock for biodiesel.
SUPPLEMENTARY MATERIALS
Supplementary Table S1
Biomass (g L−1) and lipid content (% dry biomass) of Chloroidium sp. 154-1, Chlorella sp. 154-2, and Fistulifera sp. 154-3 inoculated in increasing pH 7–11 (increments of 0.5) over 20 days of incubation (https://e-algae.org).
Supplementary Fig. S1. Sampling points along the Everglades Agricultural Area (EAA) (https://e-algae.org).
Supplementary Fig. S2. Growth based on chlorophyll content of enrichment cultures (flasks A and B) at pH 9 (A), pH 10 (B), pH 11 (C), and pH 12 (D) in modified Zarrouk’s medium over 35 days. Growth is expressed as chlorophyll a content per mL of medium (μg mL−1). Data represent the mean of three replicates (https://e-algae.org).
Supplementary Fig. S3. Culture flasks containing Chloroidium sp. 154-1 (A), Chlorella sp. 154-2 (B), and Fistulifera sp. 154-3 (C), inoculated in medium of varying pH 7–11 (increments of 0.5) on day 10 of cultivation. Showing one of four replicates (https://e-algae.org).
Supplementary Fig. S4. Biomass (g L−1 d−1) (A) and lipid (mg L−1 d−1) (B) productivity of Fistulifera sp. 154-3 over 20 days of cultivation in varying pH 7–11 (increments of 0.5). Data represent the mean of four replicates (https://e-algae.org).
Supplementary Fig. S5. Recorded pH of inoculated media with Fistulifera sp. 154-3 up to 23 days of incubation. Results are measurements of four replicates, error bars omitted for simplicity (https://e-algae.org).
Supplementary Fig. S6. Scanning electron micrographs of Fistulifera sp. 154-3 prepared by critical-point drying (A–C) and hot peroxide methods (D–H). Arrowhead depicting cell to cell contact (A), rotated side-view and top view showing thick girdle bands (B & C), top view of valve framed by fimbriate margin with central fistula opening (D), external valve showing raphe opening, curvature, and light silicification (E), internal view of top valve with distinct raphe sternum (arrowheads) (F), external view of valve with characteristic fistula (arrowhead) (G), internal valve view showing striae, protruding raphe sternum, and opening of fistula (arrowhead) (H). Scale bars represent: A–H, 1 μm (https://e-algae.org).
Supplementary Fig. S7. Comparison of lipid content (%) within dry algal weight between gravimetric and Nile red lipid quantification methods. Results indicate the mean of triplicates (https://e-algae.org).