INTRODUCTION
In marine benthic ecosystems, herbivores have a substantial effect on macroalgal community structure through their preferential feeding behavior (Paine and Vadas 1969, Worm et al. 2002, Hillebrand and Cardinale 2004). The palatability of algae and feeding preference of herbivores can lead to complex interactions between chemical or structural defense mechanisms of plants and absorption efficiency or availability of herbivores (Vadas 1977, Jormalainen et al. 2008). Therefore, herbivore feeding behavior and food availability fundamentally affect the food chain and energy flow in marine ecosystems (Huntly 1991, Burkepile and Hay 2006).
High densities of sea urchins as major mesoherbivores are a major cause for barren states in subtidal habitats (Elner and Vadas 1990, Dayton et al. 1992, Pearse 2006, Agnetta et al. 2013). Particularly, sea urchins in the family of Strongylocentrotidae are ecologically important in many coastal areas around the world because their distribution largely overlaps with kelp deforested areas (Pearse 2006, Filbee-Dexter and Scheibling 2014, Krumhansl et al. 2016, Filbee-Dexter and Scheibling 2017). In addition, their feeding preference can determine the community structure (Palacín et al. 1998, Steneck 2013), the spatial and temporal distribution of food species, and their availability in natural habitats (Vadas 1977, Harrold and Reed 1985, Agnetta et al. 2013). Food preference by sea urchins reflects their nutrient needs for somatic / gonadal growth regarding their body size (Larson et al. 1980, Lemire and Himmelman 1996, Seymour et al. 2013, Westbrook et al. 2015). Therefore, algal preference can be affected or altered by an urchin’s past feeding history (Vadas 1977, Lyons and Scheibling 2007). Flexibility in food choice has been examined in relation to maximizing caloric intake as an energy or nutrient optimization strategy (Vadas 1977, Lemire and Himmelman 1996). In addition, sea urchins move using their spines and tube feet and have a chemoreceptive ability to detect food sources around them (Mann et al. 1984, Lemire and Himmelman 1996, Lauzon-Guay and Scheibling 2008). This may suggest that sea urchins have selective foraging behaviors to increase their fitness and that their changed preference can affect the overall community structure.
The sea urchin, Mesocentrotus nudus (previously known Strongylocentrotus nudus), used in this study is widely distributed in the Northwest Pacific Ocean, including regions from Dalian, China to Primorsky Krai, Russia (Agatsuma 2001, Yurchenko and Reunov 2004). This species generally prefers brown algae such as Saccharina longissima, S. japonica, and Undaria pinnatifida (Machiguchi et al. 1994, Kawamata 1997, Kim et al. 2007). Unlike other widely studied Strongylocentrotidae sea urchins, little is known about the feeding behavior of M. nudus. This information could be particularly useful for understanding mechanistic changes of macroalgal assemblages where urchin density is a key factor such as urchin barrens in the Northwest Pacific and other regions.
In this study, we determined various feeding habits of M. nudus, including food preference and foraging behavior comparing their foraging movement with actual consumption rate. We also examined if preference could change depending on urchin’s body size and past feeding history. We discussed the feeding preference and foraging behavior of M. nudus with regard to optimal foraging theory.
MATERIALS AND METHODS
Sampling of sea urchins and algal species
The following six foliose algal species were used to determine the food preference of M. nudus: Ulva australis, Undaria pinnatifida, Sargassum confusum, Dictyopteris divaricata, Grateloupia elliptica, and Grateloupia angusta. These species commonly inhabit the central east coast of Korea (Sohn et al. 2007, Shin et al. 2008, Kim et al. 2014). They were selected as representative species for this region. Sea urchins and algae were collected by SCUBA from the subtidal zone (about 8–10 m depth) of the coast near Gangneung City, Gangwon Province, South Korea (37°50′27.8″ N, 128°52′39.1″ E). All organisms were kept in flowing seawater tanks for seven days to acclimate before they were used for experiments (see also Hernández et al. 2004).
Experimental designs and setting
This study was conducted in June and July of 2014 at the Marine Biology Center for Research and Education at Gangneung-Wonju National University located in Gangneung City. The aquaria (50 × 70 × 50 cm) used for feeding experiments were supplied with continuous flowing seawater at temperature of 17–20°C with turnover rate of 7 L per minute, which was pre-filtered from the nearby coast. Each experiment used branches or whole plants for each species except for large brown algae (S. confusum and U. pinnatifida), for which branches with blades and stipes (excluding their holdfasts and reproductive parts) were used because whole plants were too big to be used in experiments. Blotted wet weight was measured for all algae after gently shaking off excess water. Algae were tied on stainless-steel mesh and placed at the bottom of each aquarium with a plastic cable tie. Experimental aquaria contained sea urchins with six algal species. Control aquaria without sea urchins were used to measure autogenic change of algal biomass.
Experiment 1
This experiment tested primary feeding preferences of M. nudus. A total of 20 sea urchins (ten large urchins with mean test diameter of 75.3 ± 1.4 mm and weight of 101.23 ± 9.28 g and 10 small urchins with mean test diameter of 43.6 ± 1.1 mm and weight of 34.01 ± 2.56 g) were starved for 15 days (see Chang et al. 1999, James and Siikavuopio 2012), after which two individuals were placed in each of five aquaria. Sea urchins were allowed to freely feed on these six algal species (starting mean weight: U.australis, 13.83g; U.pinnatifida, 157.31 g; S. confusum, 114.84 g; D. divaricata, 8.99 g; G. elliptica, 49.7 g; and G. angusta, 5.78 g, blotted wet weight) for 12 days. Remnants of algal thalli (no species were completely eaten) were then re-weighed and compared to those in the five control aquaria to calculate the consumption rate.
Experiment 2
This experiment examined possible changes in urchin’s preference as a result of past feeding history. Because U. pinnatifida was the most preferred species from Experiment 1, ten new sea urchins were fed with U. pinnatifida only for 15 days prior to this experiment. Two sea urchins were placed in each of five experimental aquaria with five control aquaria. Physical conditions of the aquaria and days spent for the experiment were the same as in Experiment 1 except that mixed sizes (one large and one small) of urchins (mean test diameter of 61.2 ± 1.9 mm and weight of 63.96 ± 7.5 g) were used. The consumption rate was measured twice (early 6 days and later 6 days) to see possible changes in preference over time (starting mean weight: U. australis, 16.36 g; U. pinnatifida, 127.62 g; S. confusum, 73.74 g; D. divaricata, 13.92 g; G. elliptica, 48.56 g; and G. angusta, 6.78 g, blotted wet weight).
Experiment 3
Foraging movement (or behavioral choice) of M. nudus was observed using urchins with various past feeding conditions. Sea urchins (test diameter of 59.5 ± 1.9 mm, mean weight of 69.40 ± 7.1 g) were divided into three different past feeding conditions: (1) a state of starvation (starved group), (2) a state of being fed with only U. pinnatifida (Undaria-fed group), and (3) a state of being freely fed with all six algal species (mixed species-fed group). Fifteen days of such past feeding conditions were maintained prior to initiation of the experiment. Nine randomly selected individuals from each past feeding trial (total 27 individuals) were placed at the center of three aquaria of each feeding condition (three individuals for each aquarium, total nine aquaria). Six algal species were placed around the corner of the aquarium, allowing urchins to freely approach any of six choices. To measure and assess urchin’s foraging behavior, we counted the number of sea urchins approaching (within 5 cm excluding spine length) or touching any food choice every 30 min. Since M. nudus showed more active foraging behavior at night time (Hayakawa and Kittaka 1984), observation and counting were conducted for the first 4 h at night (from 12:00 am to 4:00 am). After this period, we let sea urchins freely feed on algae for 10 days. The actual consumption rate was calculated in comparison with the control group. All urchins were never used more than once throughout the series of experiment.
Data analysis
Changes in wet weight of algal food species in the experimental aquaria and the control aquaria were used to calculate the actual consumption rate using the following equation (see Molis et al. 2006, 2008):
(Fb: treatment wet weight at the beginning of experiment; Cb: control wet weight at the beginning; Fe: treatment wet weight at the end of experiment; Ce: control wet weight at the end; D: days of the experiment; N: individual number of urchins)
Analysis of variance (ANOVA) was performed to compare consumption rates between experimental groups. Before using data for ANOVA, all data were checked for normality using Kolmogorov-Smirnov test and homogeneity of variance using Levene’s test. Data were then log (x + 1) transformed to convert every dependent variable to a positive value because some calculated actual consumption rates had negative values (assumed that autogenic growth weight was higher than the amount of consumption). For the primary preference test (Experiment 1), one-way ANOVA was performed. Significance levels were marked on graphs. For effects of different feeding conditions on preference (Experiments 2 and 3), we used two-way ANOVA (Feeding × Algae). A repeated measures ANOVA was used to detect a possible preference change between early and later periods of the Undaria-fed group in Experiment 2. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Primary feeding preference and difference between large and small group
Regarding the primary feeding preference of M. nudus, the most preferred food was U. pinnatifida (2.68 g d−1), followed by G. elliptica (1.51 g d−1) and Sargassum confusum (1.31 g d−1) (Fig. 1). For the other three species, U. australis (0.25 g d−1), D. divaricata (0.13 g d−1), and G. angusta (0.04 g d−1) were consumed in very little amount. Thus, they were identified as unfavorable foods. For sea urchins with different sizes (large vs. small), there was a difference in feeding preference order between them (p = 0.012) (Fig. 2). The most preferred food by large urchins was exclusively U. pinnatifida (3.49 g d−1), while G. elliptica (1.45 g d−1) and U. pinnatifida (1.88 g d−1) were equally preferred by small urchins (Fig. 2). S. confusum was also consumed at a similar amount as G. elliptica but not much as U. pinnatifida by both large and small group. However, U. australis, D. divaricata, and G. angusta were consistently not preferred by either large or small urchins.
Preference changes according to past feeding history
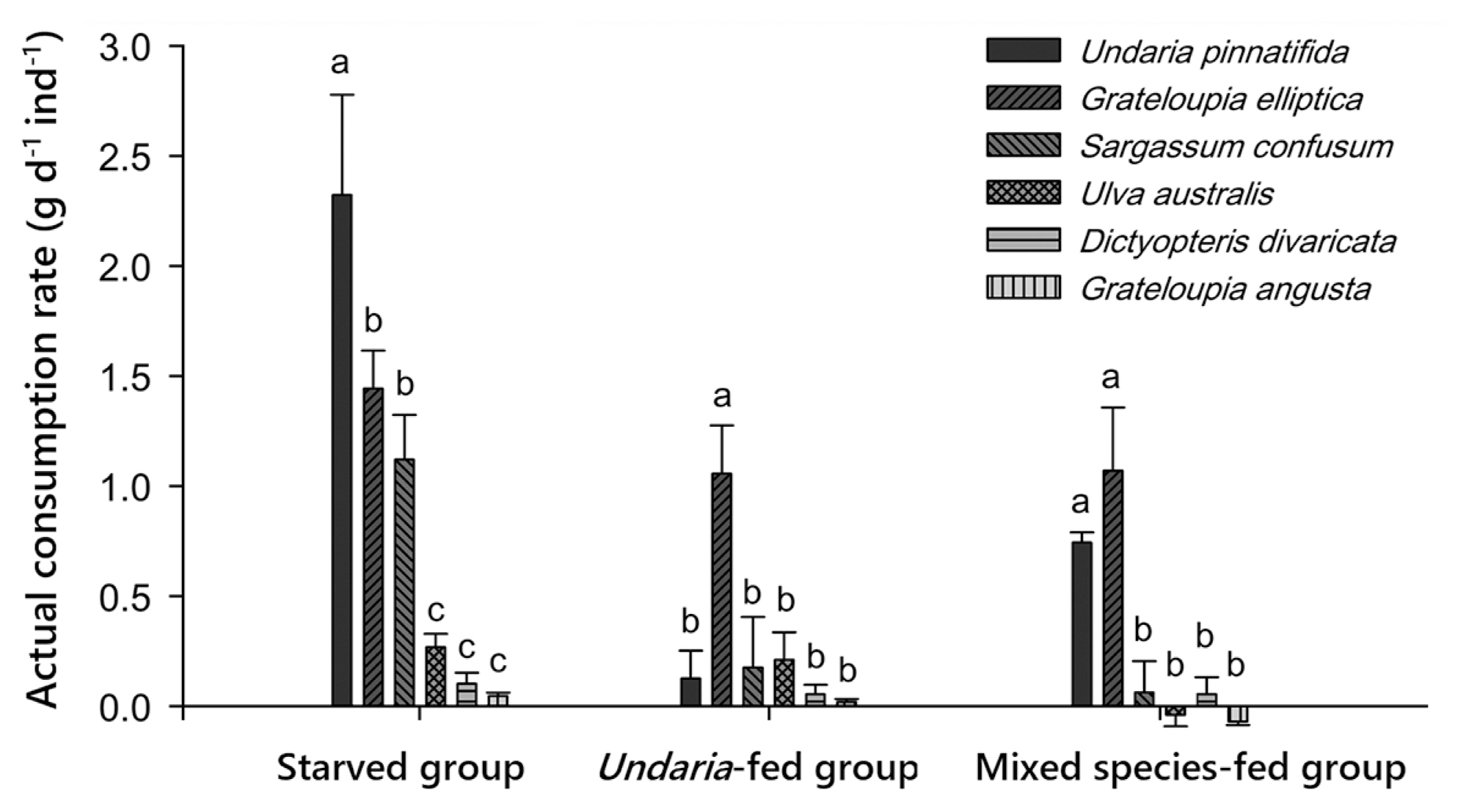
Sea urchins in the Undaria-fed group showed a remarkably reduced consumption rate for their initially most preferred U. pinnatifida compared to the starved group (Tukey’s post hoc test, p = 0.024) due to the influence of past feeding history (Table 1, Fig. 1). A similar pattern was found for another brown alga, S. confusum (Tukey’s post hoc test, p = 0.038). In particular, unlike the starved group, sea urchins in the Undaria-fed group chose G. elliptica as the most preferred food (2.06 g d−1) (Fig. 1), leaving U. pinnatifida (0.04 g d−1) and S. confusum (0.47 g d−1) as the least preferred ones among the six food species. Consumption rates for the other three algae (U. australis, D. divaricata, and G. angusta) were very low, similar to those in the starved group. Consequently, all species except G. elliptica were barely consumed by sea urchins in the Undaria-fed group.
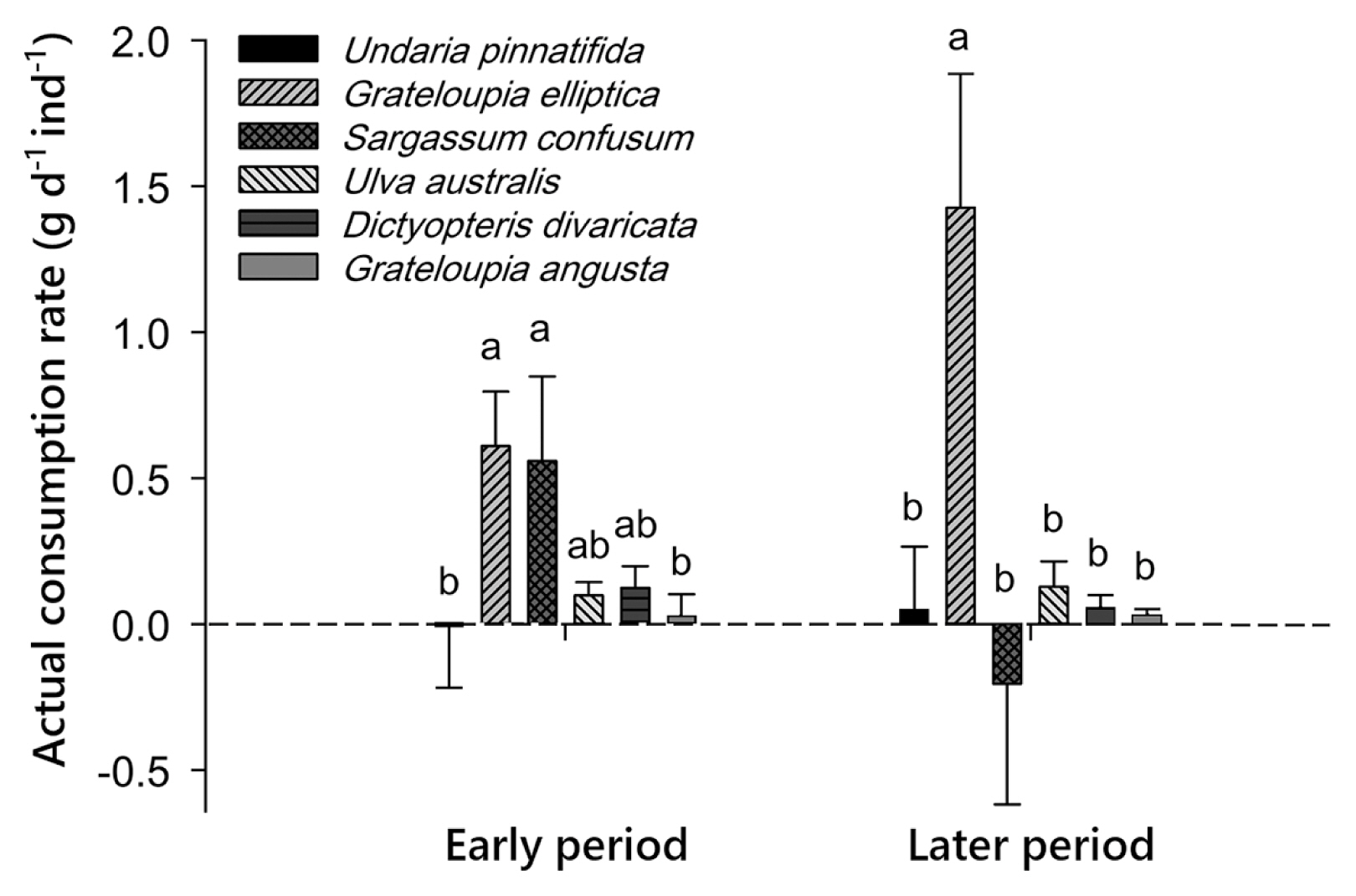
The Undaria-fed group showed a different preference according to the time elapsed after the initial feeding of Undaria. In the early six days, G. elliptica (0.61 g d−1) and S. confusum (0.56 g d−1) were almost equally preferred. However, in the later six days, only G. elliptica (1.43 g d−1) was exclusively preferred (Period × Algae, p = 0.045) (Table 2, Fig. 3). Particularly, the consumption rate of U. pinnatifida remained very low (at a rate similar to that of the least preferred group: U. australis, D. divaricata, G. angusta) for 12 days of the experimental period (Fig. 3).
Behavioral choice vs. actual consumptions under past feeding conditions
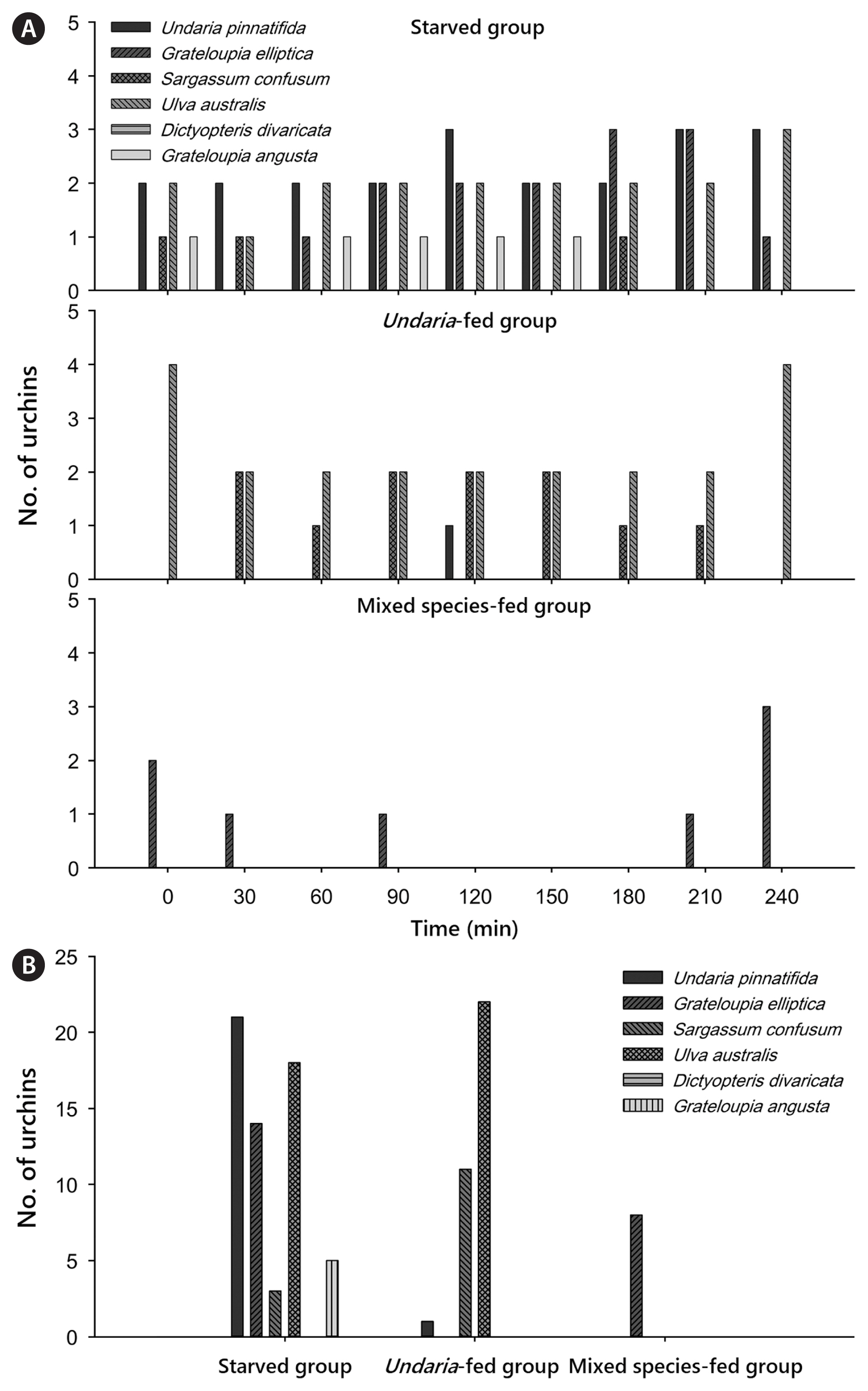
The starved group showed the most complex behavioral pattern, while the mixed species-fed group showed the simplest and inactive pattern (Fig. 4). For the starved group, U. pinnatifida and U. australis were always attracted by 2–3 sea urchins at every observation point for the entire period of 240 min. However, G. elliptica was chosen by fewer urchins after 60 min passed since the first encounter. G. angusta and S. confusum were intermittently chosen by starved urchins, whereas D. divaricata was not chosen by sea urchins (Fig. 4). Behavioral choices of urchins for the Undaria-fed group were toward U. australis and S. confusum (Fig. 4). U. australis was always chosen by 2–4 sea urchins of this group for 240 min and S. confusum was chosen by 1–2 urchins for 180 min. Not many sea urchins moved actively to chase food sources in the mixed species-fed group. Only G. elliptica was chosen intermittently by a few urchins for 240 min (Fig. 4).
Actual consumptions were not strictly matched with behavior patterns, in addition, preferred foods differed among the three groups (Table 3, Fig. 5). In the starved group, U. pinnatifida and G. elliptica were highly preferred both in behavioral choice and consumption. Although U. australis was highly attractive to urchins, it was consumed at a low rate (Fig. 5). Contrarily, S. confusum was eaten by starved urchins as much as G. elliptica, although it was ranked low in behavioral choice. Undaria-fed urchins showed a complete mismatch in that S. confusum and U. australis were the most attractive species, but the least consumed foods. On the other hand, G. elliptica was the least attractive species, but the most consumed (Fig. 5). Sea urchins of this group fed only G. elliptica for 10 days and rarely consumed other algae. Although U. pinnatifida was not attractive to sea urchins of the mixed species-fed group, this alga was the most consumed food together with G. elliptica by this group (Fig. 5). Sea urchins of this group consumed only these two food species, while they rarely consumed the other four food species.
DISCUSSION
Primary feeding preference
Starved sea urchins in this study preferred Undaria pinnatifida. Preference for brown algae by the species of Strongylocentrotidae has been reported previously (Vadas 1977, Larson et al. 1980, Harrold and Reed 1985, Kim et al. 2007). In particular, Vadas (1977) has reported that feeding preferences of three Strogylocentrotidae sea urchins (M. franciscanus, Strongylocentrotus droebachiensis, and S. purpuratus) are toward large brown algae such as Nereocystis luetkeana, Saccharina latissima, and Costaria costata, emphasizing the intake efficiency rather than the calorie content of the food source (see also Larson et al. 1980). Earlier studies have described that a high absorption efficiency of strongylocentrotid sea urchins for kelp species is due to a high level of depolymerization of digestive enzymes to alginic acid and mannitol contained in brown algae (Eppley and Lasker 1959, Boolootian and Lasker 1964). Similarly, the large brown algae, U. pinnatifida and species of Sargassum have been reported to contain large amounts of alginic acid and mannitol (Zubia et al. 2008, Borines et al. 2013, Cho et al. 2013). Therefore, the high preference for the two large browns in our study consistently supports the importance of the intake efficiency for the food choice of M. nudus.
On the other hand, D. divaricata, another brown alga, showed a significantly lower consumption rate. It belonged to the least favorable group, unlike two other brown algae. Several species of Dictyopteris (e.g., D. justii and D. polypodioides) are known to have defensive chemicals such as sulfur compounds that can deter feeding by mesoherbivores such as amphipods and fishes (Hay et al. 1988, Schnitzler et al. 2001, Teixeira et al. 2006). Furthermore, Shiraishi et al. (1991) have reported that D. divaricata used in this study shows chemical defense against M. nudus using chromazonarol. Therefore, feeding patterns of herbivores regarding brown algae appear in a somewhat complex manner. U. pinnatifida or Sargassum spp. preferred by sea urchins in this study might have a chemical defense mechanism like common brown algae, but nutritionally more beneficial due to plenty of fucoxanthin (Terasaki et al. 2009, Fung et al. 2013). In addition, the susceptibility to defense chemicals could be variable depending on herbivore species, indicating the importance of pairwise specificity for defense action and preference of generalist herbivores prevailing in marine benthic systems (e.g., Rohde et al. 2004, Molis et al. 2006, 2008).
Preference changes according to past feeding history and body size
In the Undaria-fed group, the first choice of food was switched from U. pinnatifida to G. elliptica. This preference shift of sea urchins suggests that this herbivore can temporarily pursue nutritional balance by choosing less preferred food due to its strategic flexibility in feeding patterns (Pulliam 1975, Rapport 1980). For example, Lyons and Scheibling (2007) have reported that the green sea urchin, Strongylocentrotus droebachiensis, prefers to feed kelp species Saccharina latissima over the green alga Codium fragile. However, this preference can change depending on past feeding conditions (Lyons and Scheibling 2007). What specific chemical compounds or nutrients are responsible for such temporal changes in preference of sea urchins remain unknown. Exclusive preference to a specific food source (U. pinnatifida in our case) may give urchin a clear advantage such as somatic and gonadal growth, leading to increased fitness or reproductive success (Vadas 1977, Larson et al. 1980, Lemire and Himmelman 1996). The preference change of the Undaria-fed group between early and later periods may provide a clear evidence that sea urchins can seek a different food temporally. The mechanical explanation for this preference change is unclear. It could be explained by beneficial improvement of performance with a mixed diet (Pennings et al. 1993, Cruz-Rivera and Hay 2001), supplement of insufficient nutrients (Rapport 1980), and avoiding excessive accumulation of conspecific prey’s defensive chemicals (Freeland and Janzen 1974, Vadas 1977).
Large urchins showed a stronger preference for U. pinnatifida while small urchins equally preferred U. pinnatifida and G. elliptica in our study. We assume that this subtle distinction comes from different nutritional demands for small and large urchins toward growth and reproductive success, respectively. Kawakami et al. (1998) and Kelly and Symonds (2013) have reported that fucoxanthin and β-carotene from brown algae could be advantageous egg ovulation enhancement of sea urchins. U. pinnatifida is known to contain these compounds abundantly (Kolb et al. 2004, Fung et al. 2013). Thus, large M. nudus’ increasing preference for U. pinnatifida could be related to a higher ovulation rate. On the other hand, Vadas (1977) and Larson et al. (1980) have reported that small urchins tend to choose foods advantageous for fast growing. A mixed diet may help the somatic growth of sea urchins (Scheibling and Anthony 2001, Seymour et al. 2013). These reports could explain why small urchins showed less biased preference than large urchins in our study. Therefore, preference change of sea urchins according to their body sizes might be directly related to an optimization strategy for enhancing fitness, leading to individual’s performance or potential reproductive output (see Vadas 1977, Himmelman and Nédélec 1990, Lemire and Himmelman 1996).
Foraging behavior by attractiveness vs. actual consumption
In Experiment 3, sea urchins’ foraging behavior by attractiveness of food species was largely consistent with their final consumption amounts, although they exhibited various exploratory approaches even to unpalatable foods at the beginning. These foraging behaviors also differed depending on dietary histories, indicating that urchins’ food detecting responses were quite fast and sensitive. Interestingly, a few food species such as U. australis or G. angusta were chosen by sea urchins, although they were not consumed significantly. This may be explained by the fact that sea urchins can detect prey at a short distance by moving randomly and taste first and seek for another palatable prey if it is not. It has been reported that sea urchins S. droebachiensis and M. nudus generally move randomly or stochastically when they are foraging (Hayakawa and Kittaka 1984, Lauzon-Guay et al. 2006, Dumont et al. 2007). Dumont et al. (2007) have suggested that such random movement could be an appropriate behavior to enhance their chance to find food sources for sea urchins with limited perceptive ability. Likewise, sea urchins in this study seemed to move randomly at the first few hours. However, their final consumption amounts were largely coincident to their behavioral choice, indicating that this foraging behavior could be correlated with their actual consumption. Sea urchins’ movement tends to be complex and random, but changeable depending on their dietary state in natural habitats such as barrens and algal beds. As shown partly in our indoor aquarium study, fully fed urchins in algal beds showed less active foraging movements. On the other hand, starved urchins in barrens showed more active and destructive grazing that might cause the barren state recycled (see Garnick 1978). This switching grazing modes of sea urchins might be one of key factors determining the community state where they inhabit as a part of a feedback mechanism (Filbee-Dexter and Scheibling 2014).
In summary, the primary feeding preference of sea urchin, M. nudus, was toward a brown alga U. pinnatifida. However, M. nudus showed preferential flexibility depending on their past feeding histories and body size. Additionally, their movement complexity toward food choice was strongly affected by past feeding histories. Our results indicate that M. nudus as a generalist herbivore can show plasticity and flexibility of feeding and foraging behaviors. This study provides experimental evidence of complex feeding patterns of a common sea urchin. It contributes to the feeding ecology of urchins and better understanding of mechanistic changes in macroalgal communities where urchin grazing is a major driving force.