ABSTRACTThe hepatoprotective effect of liposoluble portion of Pyropia yezoensis (PYLP) was investigated against alcohol-induced liver injury in mice. Fatty acids were predominant in PYLP obtained from hexane fraction of 70% EtOH extract after ultrasonication. In particular, polyunsaturated fatty acids such as eicosapentaenoic acid and linoleic acid accounted for 56.91% of the total lipids. PYLP significantly reduced liver damage induced by the alcohol treatment in mice. PYLP treatment increased the activity of antioxidant enzymes including superoxide dismutase, catalase, and glutathion peroxidase by reducing thiobarbituric acid reactive substances. Histological observations showed that PYLP minimizes damage to living tissue induced by alcohol treatment by modulating the expression level of proteins involved in the anti-apoptotic signaling pathway. Our results suggest that PYLP, rich in polyunsaturated fatty acids extracted from the red alga P. yezoensis, will be useful as a potential liver protectant in the hangover industry.
INTRODUCTIONLiver disorders are caused by various agents such as alcohol, environmental toxins and viruses, and they remain one of the major threats to public health (Suresh and Asha 2008). Alcohol abuse has widespread negative impacts on human health and social economy. Chronic exposure to alcohol causes significant damages to the liver and can result in microvascular steatosis, apoptosis, necrosis, inflammation, hepatic fibrosis, and ultimately, cirrhosis (Lambert et al. 2003, Lu et al. 2015). Therefore, there are ongoing researches on potential pharmacotherapeutic agents to cure alcoholic hepatitis. Previously reported therapeutic agents for alcoholic hepatitis, such as pentoxifylline, infliximab, and etanercept are associated with side effects including an increase in infections and death (Santos Rocha et al. 2012). Undoubtedly, natural products could be alternatives to synthetic medicines for the prevention of alcohol-induced disorders (Lin et al. 2014).
Marine algae are rich in functional ingredients such as polysaccharides, polyphenols, phytosterols, and carotenoids (Lee et al. 2013, 2014, 2020, Kim et al. 2014, 2016, Shanura Fernando et al. 2017, Asanka Sanjeewa et al. 2019, Ding et al. 2019, Dai et al. 2020). Pyropia species, belonged to Bangiaceae family which are the most important perennial edible marine red algae, are cultivated and consumed in East and Southeast Asian countries such as Korea, Japan, and China. P. yezoensis is well known for its nutritional value. It has been used as a food source and a traditional medicine material for centuries in Asia (Kazłowska et al. 2013). Because it is rich in biological components such as sterols, dietary fibers, taurine, polyunsaturated fatty acids, carotenoids, and mycosporine-like amino acids including porphyra-334. It is also rich in minerals, vitamins, and proteins (Isaka et al. 2015, Zang et al. 2020). Porphyran, a water soluble portion, is a functional sulfated polysaccharide obtained from P. yezoensis and has shown strong protective effects in mice against carbon tetrachloride (CCl4)-induced hepatotoxicity, caused by inflammatory reactions, free radicals, and lipid peroxidation (Guo et al. 2007). However, the hepatoprotective effect of a liposoluble portion in P. yezoensis (PYLP) has not been reported. In this study, therefore, the in vivo protective effect of PYLP against the alcohol-damaged liver was studied.
MATERIALS AND METHODSReagentsAll solvents used for sample preparation were of analytical grade (Daejung Chemicals & Metals Co., Seoul, Korea). High-performance liquid chromatography (HPLC) grade solvents were purchased from Burdick & Jackson (Muskegan, MI, USA). Arylesterase/Paraoxonase assay kit was purchased from ZeptoMetrix Corporation (Stamar, Dabrowa Gornicza, Poland). Antibodies against p53, Bcl-xL, Bax, and β-actin were purchased from Cell Signaling Technology (Bedford, MA, USA). All other chemicals and reagents were used as analytical grade.
Collect of Pyropia yezoensis
P. yezoensis was collected at near coast of Wondo on November 2014 and inspected by comparing with specimen (No. 131) supported by Dr. Lee, Ki-Wan retired from Jeju National University, then P. yezoensis was washed three times by tap water to the derived foreign matters which dried by freeze dryer (PVTFD 10A; Ilshin Bio Base Co., Ltd., Dongducheon, Korea), kept at −4°C until its usage.
Preparation of polyunsaturated fatty acids-rich PYLPIn order to prepare PYLP, firstly, a 70% ethanol extract (85 g) was manufactured by three times extracting from dried P. yezoensis (600 g) in ultrasonication. And the dried extract powder was prepared through steps by a rotary evaporator (N-1000; Tokyo Rikikai Co., Ltd., Tokyo, Japan) and freeze dryer (SFDSM06; Samwon Freezing Exgineering Co., Busan, Korea), respectively. Finally, PYLP (12.56 g) was obtained from suspension fraction using n-hexane and distilled water (1 : 1, v/v), and then kept in refrigerator (at −20°C) after drying.
Analysis of biological components in PYLPFatty acidsPYLP was dissolved in n-hexane and 0.2 mL of 2 M methanolic-NaOH was added. Then, the mixture was shaken and kept at 50°C for 30 s, and then 0.2 mL of 2 M HCl in methanol was added and shaken to neutralize. The mixture was separated by centrifugation at 700 ×g for 5 min, the upper n-hexane layer was collected and concentrated. A 0.5 m thickness PEG-20 M liquid phase-coated 40 m × 1.2 mm diameter G-300 column (Chemicals Evaluation and Research Institute, Saitama, Japan) was used for analysis by connecting it to a Hitachi 263 gas chromatograph (Hitachi Co., Ltd., Ibaraki, Japan) equipped with a flame ionization detector. The temperatures of the column, detector, and injection port were set to 190, 240, and 250°C, respectively.
Total sterolsThe conditions used for saponification and extraction are modified from those proposed by Sánchez-Machado et al. (2004). PYLP (1.00 ± 0.01 g) was weighed out in an Erlenmeyer flask and saponified by refluxing with 27 mL of 1 M ethanolic KOH for 30 min with a constant shaking under the temperature of 80°C. After cooling to ambient temperature, the mixture was transferred to a separation funnel to extract the non-saponifiable fraction with total 40 mL including 20 mL of DW + the mixture and 20 mL of hexane (agitation washing for 3 min each time). The hexane containing the sterols was evaporated to dryness in a rotavapor at 40°C. The quantification of total sterol was evaluated by Livermann-burchard test. The residue was dissolved in 3 mL of mobile phase (30 : 70, methanol : acetonitrile, v/v), then filtered through a 0.45 μm membrane. A 10 μL of this extract was injected into the HPLC column (Waters Corporation, Milford, MA, USA). The HPLC-UV system comprised a liquid chromatograph equipped with a 515 HPLC pump, and a photo diode array detector (2998 PDA detector), controlled by Empower software (Waters Corporation). Temperature was controlled at 50 ± 0.1°C with a column heater (Waters Corporation). Separation was performed in a SunFire C18 5 μm column (4.6 × 250 mm i.d.; Waters Corporation), with 30 : 70 (methanol : acetonitrile, v/v) at 1.0 mL min−1 as a mobile phase. The eluted solution was detected at 200 nm wavelength. Each sterol from PYLP was identified by the report of Sánchez-Machado et al. (2004).
β-CaroteneThe analysis for quantification of β-carotene is modified from those proposed by Ahamad et al. (2007). Standard of β-carotene (1 g enclosed in vial) was purchased from Merck KGaA (Darmstadt, Germany). Stock solution of β-carotene was prepared by taking 10 mg in 100 mL n-hexane. The concentration of stock solution was equal to 100 ppm. HPLC system and chromatographic condition was Perkin Elmer HPLC programme containing LC-1000 pump (Isocratic), equipped with C18 column and LC 250 UV/VIS detector (PerkinElmer Inc., Waltham, MA, USA). HPLC was calibrated by running mobile phase (acetonitrile : dichloromethane : methanol, 70 : 20 : 10) at the rate of 1 mL min−1. The wave length was fixed at 452 nm. A 10 μL of β-carotene or PYLP was injected into the HPLC column. The concentration of the β-carotene in PYLP was calculated using the β-carotene standard curve.
Animal and experimental designMale BALB/c mice (6-week-old, 23–26 g body weight) were purchased from Dae Han Bio Link Co., Ltd. (Eumseong, Korea). All these mice raised in the specific pathogen free clean room under conditions of 21 to 25°C and a 12 h dark : light cycle, and had free access to laboratory chow and tap water. Jeju National University’s Institutional Animal Care and Use Committee approved the protocol for the animal study, and the animals were cared for in accordance with the “Guidelines for Animal Experiments” set by the university.
Balb/c mice were randomly divided into four groups as following: normal group (saline applied mice, each 100 μL, n = 6), alcohol only (alcohol 3 g kg−1 mice and saline co-applied mice, each 100 μL, n = 10), PYLP + alcohol (alcohol 3 g kg−1 mice and PYLP 25 mg kg−1 mice co-applied mice, each 100 μL, n = 10), and silymarin + alcohol (alcohol 3 g kg−1 mice and silymarin 50 mg kg−1 mice co-applied mice, each 100 μL, n = 6). Alcohol, PYLP, and silymarin were daily oral administrated to mice using oral-zoned needle connected to a 1 mL syringe for 10 days. During the experiment period, the body weight and the survival rate of mice were daily measured. On the 10th day, the mice were dissected after the measurement of body weight. Additionally, blood samples were collected into heparin-coated tubes to determine biochemical parameters and liver tissues were stored at −70°C until analysis.
Detection of biochemical indicators in bloodThe serum activities of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) were determined according to the Reitmen-Frankel method (Reitman and Frankel 1957) using an enzyme assay kit (Asan Pharmaceuticals, Hwasung, Korea). The total cholesterol content in the serum was determined using a commercial available kit (Asan Pharmaceuticals) according to the method of Allain et al. (1974). Also, lipid peroxidation concentrations in serum was determined by measuring thiobarbituric acid reactive substances (TBARS) based on the method of Ohkawa et al. (1979). The TBARS concentration was expressed as nmol of malondialdehyde (MDA) per mL serum.
Measurement of the activities of antioxidant enzymesThe liver tissue was homogenized by adding five volumes of ice-cold homogenization buffer (250 mM sucrose, 50 mM Tris-HCl, pH 7.4, 1 mM EDTA). After the homogenized liver tissue was centrifuged at 1,000 ×g for 10 min and the supernatant was centrifuged again at 12,000 ×g for 30 min. The final supernatant protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as the protein standard. The activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured using a commercially available kit (Biovision, Milpitas, CA, USA). The SOD activity was estimated spectrophotometrically at 450 nm while monitoring the inhibition of superoxide anions by the xanthine/xanthine oxidase system and the enzyme activity was expressed as U mg−1 protein. The CAT activity was determined as the degradation rate of H2O2, and the degradation rate was measured at 570 nm. In the GPx activity assay, enzymatic reactions involving NADPH, reduced glutathione (GSH) and glutathione reductase, was initiated with the addition of cumene hydroperoxide. The change of absorbance was monitored at a wavelength of 340 nm and the activity was given as mU mg−1 protein.
Western blot testThe liver tissue was homogenized in RIPA buffer (Sigma Chemical Co., St. Louis, MO, USA). After centrifugation at 12,000 ×g for 10 min, the protein concentration of the lysates was measured by BCA protein assay kit (Merck KGaA). The lysates containing 50 μg of protein were applied to 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. After blocking with a solution containing 5% skim milk in TBS containing 0.1% Tween 20 (TBS-T) for 2 h to prevent non-specific binding of antibody, the membranes were incubated with primary antibodies against Bax, p53, Bcl-xL, and β-actin in TBS-T for overnight at 4°C. The membranes were then washed with TBS-T and incubated with secondary antibodies. Signals were developed using chemiluminescence Western blotting detection kit, and imaged on Davinch-Chemi imaging system (Core Bio, Seoul, Korea).
Hematoxylin and eosin stainingLiver tissues were fixed in 10% (v/v) phosphate buffer formalin and embedded in paraffin wax. The paraffin sections (4–6 μm thick) were cut, and each section was stained with hematoxylin and eosin, and histological analysis was performed under light microscope (DP70; Olympus Optical Co., Tokyo, Japan).
RESULTS AND DISCUSSIONAlcoholic liver diseases remain a global health problem, but there are still no effective therapies. It is well established that oxidative stress induced by ethanol consumption plays a key role in the progression of liver damage. Therefore, the use of natural antioxidants is an effective approach to inhibit oxidative stress and alleviate ethanol-induced hepatotoxicity (Chen et al. 2016). For this reason, we investigated the water-insoluble extract of P. yezoensis, which contains abundant polyunsaturated fatty acids as a potential natural protector agent against hepatic injury induced by ethanol in mice.
PYLP was analyzed for various chemical components such as total fatty acids, β-carotene, and total sterols, and they indicated 84.52, 10.01, and 2.30%, respectively (Table 1) to confirm target component for anti-alcoholic hepatic disease. As the result of fatty acid composition, PYLP contained several major fatty acids such as 54.12% eicosapentaenoic acid (EPA; C20:5), 21.07% palmitic acid (C16:0), 6.43% tricosanoic acid (C23:0), and 4.99% linoleic acid (LA; C18:2) (Table 2). And the sterols were composed of 72.10% of desmosterol, 10.01% of cholesterol, and 17.31% of mixture of stigmasterol and campesterol (data not shown). Shin et al. (2013) and Parrish (2009) have reported that EPA is the dominant fatty acid in P. yezoensis, forming as much as 50% of the total fatty acid content. In our results also showed that PYLP contains high fatty acids content (84.52%) and especially, exhibited 56.2% of polyunsaturated fatty acids. Also, this study showed high EPA content of 54.12%.
Polyunsaturated fatty acids such as EPA and LA play a significant role in mitigating cardiovascular disease (Lemaitre et al. 2003, Yokoyama et al. 2007, Lluís et al. 2013), moderating tissue inflammation (Uauy and Valenzuela 2000, Ruxton et al. 2004), and contributing to the development of the nervous systems in humans (Burdge 1998). Especially, EPA possesses various biological effects including antioxidant, anticancer, anti-inflammatory, and vascular effects. Thus, EPA markedly alleviated valproate-induced hepatotoxicity, oxidative stress, and inflammation, while enhancing the anticonvulsant effects of valproate without altering its clearance (El-Mowafy et al. 2011). β-Carotene showed protective effect against methotrexate and fenitrothion induced oxidative liver damage through decrease of reactive oxygen species (ROS) (Hashim and Weshahy 2002, Vardi et al. 2010). Especially Peng et al. (2013) demonstrated that low dose of β-carotene provides protection against hepatic injury. And phytosterols such as stigmasterol and campesterol were reported on their decrease effect of liver inflammation (Plat et al. 2014). Therefore, we expected the hepatic protective effect of liposoluble portion of P. yezoensis contained these bio-active components on alcohol-induced liver damage. Especially, liposoluble portion named polyunsaturated fatty acids-rich liposoluble portion due to the highest amount of polyunsaturated fatty acids and polyunsaturated fatty acids was considered as main bioactive component.
The weights and survival rates in mice were measured to evaluate the protective effect of PYLP on alcohol-damaged mice and showed in Fig. 1. It was observed that normal (= non-treatment group), alcohol only, PYLP + alcohol or silymarin + alcohol (positive control) treatments did not affect to the weights of the mice. However, alcohol critically decreased the survival rate at 3 days after treatment, whereas PYLP maintained a 100% survival rate until 8 days. At day 9, the PYLP + alcohol group showed a decrease of 20% in survival rate; however, the treatment with silymarin did not result in death of the mice at any stage during the experiments.
Table 3 showed the results of alcohol only, PYLP + alcohol, and silymarin + alcohol treatments on the levels of GOT (also known aspartate aminotransferase [AST]), GPT (also known as alanine aminotransferase [ALT]), total cholesterol and MDA in serum of mice. GOT and GPT are found in the liver, blood, heart, muscle, pancreas, and kidneys as major indicators of organ damage and liver disease (Ren et al. 2009, Lin et al. 2010). In this study, the alcohol-treated mice showed higher levels of GOT and GPT in the serum than those of the control group, whereas the treatments with PYLP as well as silymarin significantly decreased those levels. Total cholesterol in serum is a possible indicator for the protective effect of PYLP against alcoholic liver injury (Zhang et al. 2014). From the results, it was observed that total cholesterol in the serum was significantly decreased in PYLP + alcohol group, compared with the alcohol only treated group (p < 0.01). In the previous report by Guo et al. (2007), porphyran (an acidic polysaccharide), one of the main components of the water-soluble extract of P. yezoensis, showed hepatoprotective effect against CCl4-treated mice. In the study, ALT and AST levels in the liver homogenate of porphyran + CCl4 induced mice were decreased by 11.80 and 21.70%, respectively at a dose of 6.25 mg kg−1. The MDA level in porphyran + CCl4-induced mice decreased by 25.8%. In recent, Choi et al. (2016) reported that glycoproteins from P. yezoensis (PYGP) protect chronic ethanol-induced hepatotoxicity in rats. As the study, PYGP showed the significant hepatoprotective effect by decreasing both GOT and GTP, and increasing the activations of GSH, GSH-px, and catalase in 300 mg kg−1 of rat. From the results, PYLP might be considered to be a useful hepatoprotective agent.
Chronic ethanol exposure impairs endogenous non-enzymatic (e.g., GSH) and enzymatic (e.g., superoxide dismutase [SOD] and GPx) antioxidant systems that protect hepatocytes against oxidative damage (Cao et al. 2015). Thus, a large amounts of ROS generated by ethanol stimulate the peroxidation of low-density lipoprotein cholesterol which causes a significant increase in MDA formation. Therefore, MDA is often used as an indicator of oxidative status (Harris et al. 2015, Macan et al. 2015). As shown in Fig. 2A–C, there were significant reductions in the activations of the enzymes in livers of the mice administered with alcohol, whereas PYLP significantly increased them (p < 0.01). And the alcohol treatment also significantly increased the level of TBARS, whereas PYLP inhibited the cholesterol peroxidation in alcohol treated mice (Fig. 2D). Silymarin and PYLP exhibited a similar protective effect against alcohol-induced oxidative stress.
Generally, alcohol has an adverse effect on liver tissues (Matsumoto et al. 2016); therefore, a histopathological change in liver tissue is key in evaluating the protective effect of PYLP against alcohol-induced liver injury. As shown in Fig. 3, livers of the normal mice showed a normal architecture of the hepatic cells, bile lobes, and periportal regions. The hepatic tissues from the alcohol only groups showed hepatocyte degeneration manifested by vacuolization and hemorrhagic lesions. However, the liver tissues from the silymarin and alcohol co-administered groups were less damaged with hepatic degeneration. In addition, the PYLP-treated group also showed intact liver histology with no damaged hepatocytes and portal vasculature, unlike the alcohol only group.
Oxidative stress induced by ethanol or other stimuli generates apoptosis via expression of p53 and Bcl-2 family proteins (Liu et al. 2008, Behera et al. 2014, Chen et al. 2016). PYLP decreased the expression of the pro-apoptotic proteins, p53 and Bax, whereas increased the expression of anti-apoptotic protein, Bcl-xL. The results shown in Fig. 4 indicate that ethanol increased the expression of the pro-apoptotic protein, p53, and Bax proteins, while PYLP decreased the expression of p53 and Bax, and increased the expression of anti-apoptotic protein and Bcl-xL. However, PYLP showed a relatively lower anti-apoptotic effect than silymarin. The expression of anti-apoptotic protein, Bcl-xL was slightly elevated in the PYLP-treated mice than in the alcohol only group. Collectively, PYLP inhibited apoptosis of hepatocytes via activation of p53 and Bax in the mice administered alcohol.
CONCLUSIONAlcohol occurs liver injury by various mechanisms, such as oxidative stress and apoptosis signaling pathway. Our results indicate that PYLP reduces alcohol-induced liver injury in vivo by reducing total cholesterol, inhibiting ROS generation, increasing activities of antioxidant enzyme, reducing MDA formation and anti-apoptosis pathway. These effects are due to the abundant functional components of PYLP such as the polyunsaturated fatty acids. Finally, this study suggests that PYLP is more beneficial to human health for protecting alcohol-damaged liver injury.
ACKNOWLEDGEMENTSThis research was supported by Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS) (213004-04-2-SB930), and this work was also supported by Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry for Health and Welfare, Korea (HI14C1135 & HI15C0987).
Fig. 1Protective effect of liposoluble portion of Pyropia yezoensis (PYLP) on alcohol-induced toxicity in mice. (A) Body weight. (B) Survival rate. BALB/c mice were randomly divided into four groups as following: normal group (saline applied mice, each 100 μL, n = 6), alcohol only (alcohol 3 g kg−1 mice and saline co-applied mice, each 100 μL, n = 10), PYLP + alcohol (alcohol 3 g kg−1 mice and PYLP 25 mg kg−1 mice co-applied mice, each 100 μL, n = 10), and silymarin + alcohol (alcohol 3 g kg−1 mice and silymarin 50 mg kg−1 mice co-applied mice, each 100 μL, n = 6). Alcohol, PYLP, and silymarin were daily oral administrated to mice using oral-zoned needle connected to a 1 mL syringe for 10 days. During the experiment period, the body weight and the survival rate of mice were daily measured. 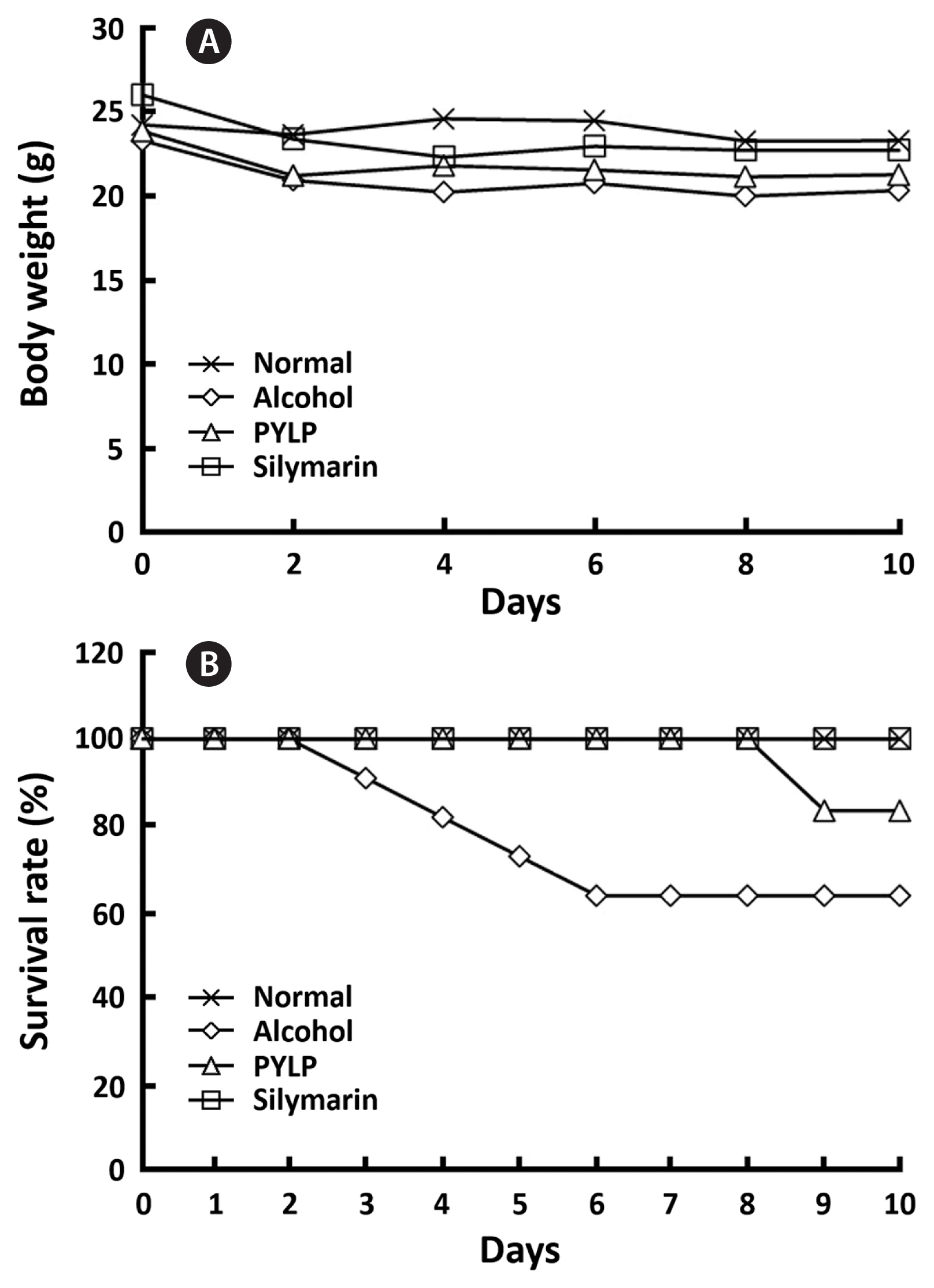
Fig. 2Protective effect of liposoluble portion of Pyropia yezoensis (PYLP) on alcohol-induced oxidative stress in liver. (A) Superoxide dismutase (SOD) activity. (B) Catalase (CAT) activity. (C) Glutathione peroxidase (GPx) activity. (D) Thiobarbituric acid reactive substances (TBARS) (malondialdehyde [MDA]). Each experiment was performed in four groups as follows: normal (= non-treatment), alcohol only, PYLP + alcohol, and silymarin + alcohol (positive control) treatments. Data are expressed as mean ± standard error (n = 3). Different letters indicate significant differences (p < 0.05) according to Duncan test. 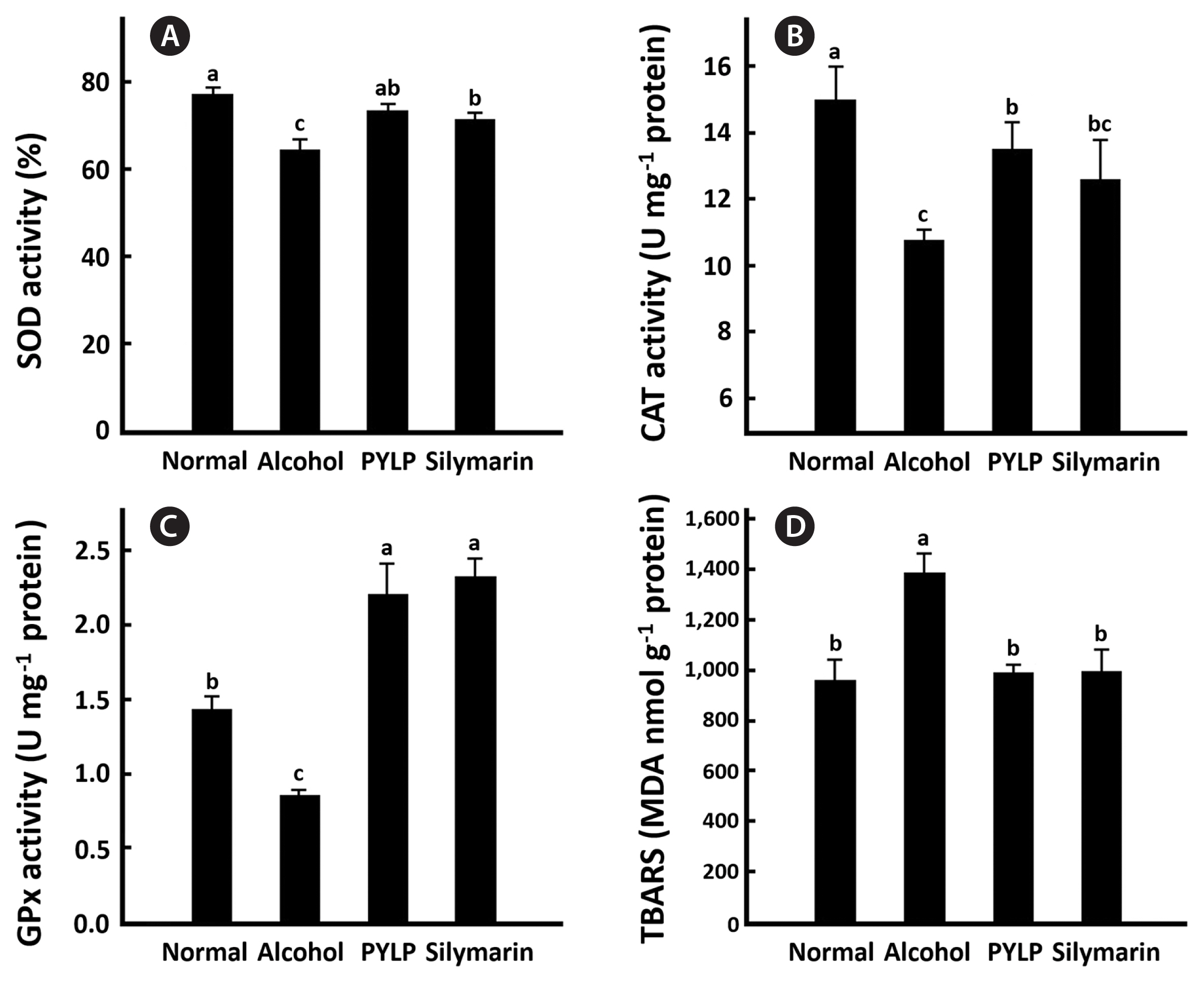
Fig. 3Histologic results of tissues in alcohol-induced mice. The liver tissue sections were stained with hematoxylin and eosin (H&E staining, ×100). (A) Normal group (non-treatment). (B) Alcoholic group (EtOH). (C) Alcoholic plus liposoluble portion of Pyropia yezoensis group. (D) Alcoholic plus silymarin group. Scale bars represent: A–D, 10 μm. 
Fig. 4Liposoluble portion of Pyropia yezoensis (PYLP) reduced apoptosis generated in liver by alcohol-induced oxidative stress. Densitometry analysis (A) and relative intensity (B) of p53, Bcl-xL, and Bax compared to β-actin. Each experiment was performed in four groups as follows: normal (= non-treatment), alcohol only, PYLP + alcohol, and silymarin + alcohol (positive control) treatments. Values are expressed as mean ± standard deviation. Statistical analysis was carried out with the Tukey’s test (***p < 0.005). 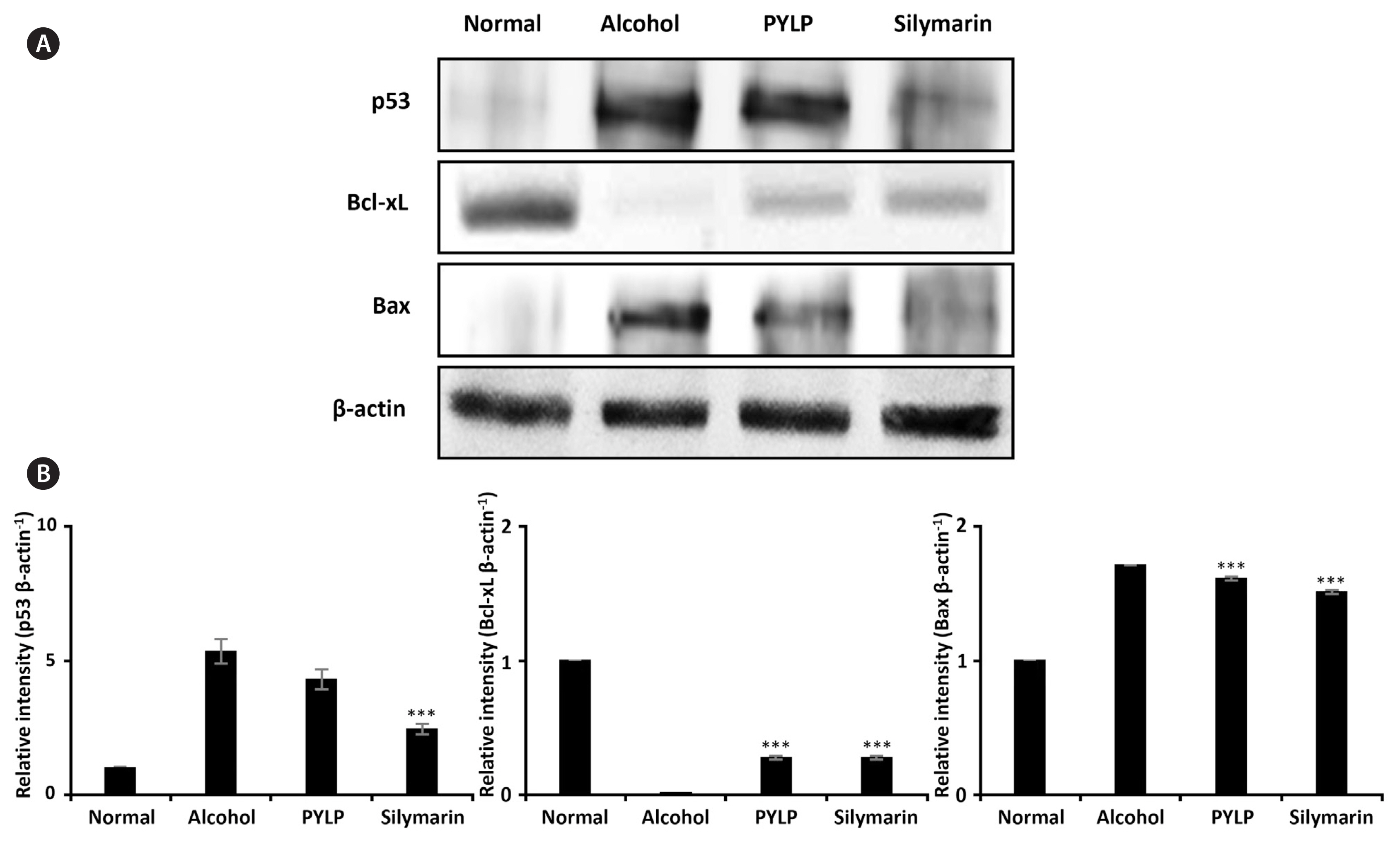
Table 1Comparison of the components contents in PYLP
Table 2Comparison of fatty acids composition in PYLP Table 3Comparison of GOT, GPT, total cholesterol, and malondialdehyde in serum of mice REFERENCESAhamad, MN., Saleemullah, M., Shah, HU., Khalil, IA. & Saljoqi, AUR. 2007. Determination of beta carotene content in fresh vegetables using high performance liquid chromatography. Sarhad J Agric. 23:767–770.
Allain, CC., Poon, LS., Chan, CSG., Richmond, W. & Fu, PC. 1974. Enzymatic determination of total serum cholesterol. Clin Chem. 20:470–475.
Asanka Sanjeewa, KK., Fernando, IPS., Kim, S-Y., Kim, W-S., Ahn, G., Jee, Y. & Jeon, Y-J. 2019.
Ecklonia cava (Laminariales) and Sargassum horneri (Fucales) synergistically inhibit the lipopolysaccharide-induced inflammation via blocking NF-κB and MAPK pathways. Algae. 34:45–56.
Behera, B., Mishra, D., Roy, B., Devi, KSP., Narayan, R., Das, J., Ghosh, SK. & Maiti, TK. 2014.
Abrus precatorius agglutinin-derived peptides induce ROS-dependent mitochondrial apoptosis through JNK and Akt/P38/P53 pathways in HeLa cells. Chem-Biol Interact. 222:97–105.
Bradford, MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
Burdge, GC. 1998. The role of docosahexaenoic acid in brain development and fetal alcohol syndrome. Biochem Soc Trans. 26:246–252.
Cao, Y-W., Jiang, Y., Zhang, D-Y., Zhang, X-J., Hu, Y-J., Li, P., Su, H. & Wan, J-B. 2015. The hepatoprotective effect of aqueous extracts of Penthorum chinense Pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 6:1510–1517.
Chen, L-Y., Chen, Q., Cheng, Y-F., Jin, H-H., Kong, D-S., Zhang, F., Wu, L., Shao, J-J. & Zheng, S-Z. 2016. Dallyl trisulfide attenuates ethanol-induced hepatic steatosis by inhibiting oxidative stress and apoptosis. Biomed Pharmacother. 79:35–43.
Choi, J-W., Kim, I-H., Kim, Y-M., Lee, M-K., Choi, Y-H. & Nam, T-J. 2016. Protective effect of Pyropia yezoensis glycoprotein on chronic ethanol consumption-induced hepatotoxicity in rats. Mol Med Rep. 14:4881–4886.
Dai, YL., Kim, GH., Kang, M-C. & Jeon, Y-J. 2020. Protective effects of extracts from six local strains of Pyropia yezoensis against oxidative damage in vitro and in zebrafish model. Algae. 35:189–200.
Ding, Y., Kim, SH., Lee, JJ., Hong, JT., Kim, EA., Kang, DH., Heo, S-J. & Lee, S-H. 2019. Anti-melanogenesis activity of Ecklonia cava extract cultured in tanks with magma seawater of Jeju Island. Algae. 34:177–185.
El-Mowafy, AM., Abdel-Dayem, MA., Abdel-Aziz, A., El-Azab, MF. & Said, SA. 2011. Eicosapentaenoic acid ablates valproate-induced liver oxidative stress and cellular derangement without altering its clearance rate: dynamic synergy and therapeutic utility. Biochim Biophys Acta. 1811:460–467.
Guo, T-T., Xu, H-L., Zhang, L-X., Zhang, J-P., Guo, Y-F., Gu, J-W. & He, P-M. 2007.
In vivo protective effect of Porphyra yezoensis polysaccharide against carbon tetrachloride induced hepatotoxicity in mice. Regul Toxicol Pharmacol. 49:101–106.
Harris, PS., Roy, SR., Coughlan, C., Orlicky, DJ., Liang, Y., Shearn, CT., Roede, JR. & Fritz, KS. 2015. Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biol. 6:33–40.
Hashim, EF. & Weshahy, K. 2002. Analytical and microscopical studies on the protective effect of ascorbic acid (vitamin C) and beta-carotene against the toxicity induced by fenitrothion on the liver of female albino rats. Egypt J Hosp Med. 7:1–27.
Isaka, S., Cho, K., Nakazono, S., Abu, R., Ueno, M., Kim, D. & Oda, T. 2015. Antioxidant and anti-inflammatory activities of porphyrin isolated from discolored nori (Porphyra yezoensis). Int J Biol Macromol. 74:68–75.
Kazłowska, K., Lin, H-TV., Chang, S-H. & Tsai, G-J. 2013.
In vitro and in vivo anticancer effects of sterol fraction from red algae Porphyra dentata. Evid-Based. Complement Alternat Med. 2013. 493869 pp.
Kim, H-H., Kim, H-S., Ko, J-Y., Kim, C-Y., Lee, J-H. & Jeon, Y-J. 2016. A single-step isolation of useful antioxidant compounds from Ishige okamurae by using centrifugal partition chromatography. Fish Aquat Sci. 19:22 pp.
Kim, S., You, DH., Han, T. & Choi, E-M. 2014. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J Photochem Photobiol B. 141:301–307.
Lambert, JC., Zhou, Z., Wang, L., Song, Z., Mcclain, CJ. & Kang, YJ. 2003. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 305:880–886.
Lee, J-H., Ko, J-Y., Oh, J-Y., Kim, C-Y., Lee, H-J., Kim, J. & Jeon, Y-J. 2014. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 158:433–437.
Lee, J-H., Ko, J-Y., Samarakoon, K., Oh, J-Y., Heo, S-J., Kim, C-Y., Nah, J-W., Jang, M-K., Lee, J-S. & Jeon, Y-J. 2013. Preparative isolation of sargachromanol E from Sargassum siliquastrum by centrifugal partition chromatography and its anti-inflammatory activity. Food Chem Toxicol. 62:54–60.
Lee, SM., Kim, NH., Ji, YK., Kim, YN., Jeon, YJ., Heo, JD., Jeong, EJ. & Rho, J-R. 2020. Sulfoquinovosylmonoacylglycerols regulating intestinal inflammation in co-culture system from the brown alga Turbinaria ornata
. Algae. 35:201–212.
Lemaitre, RN., King, IB., Mozaffarian, D., Kuller, LH., Tracy, RP. & Siscovick, DS. 2003. n-3 polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the cardiovascular health study. Am J Clin Nutr. 77:319–325.
Lin, J-D., Lin, P-Y., Chen, L-M., Fang, W-H., Lin, L-P. & Loh, C-H. 2010. Serum glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res Dev Disabil. 31:172–177.
Lin, X., Zhang, S., Huang, R., Wei, L., Tan, S., Liang, S., Tian, Y., Wu, X., Lu, Z. & Huang, Q. 2014. Helenalin attenuates alcohol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and suppressing HSC activation. Fitoterapia. 95:203–213.
Liu, B., Chen, Y. & Clair, DKS. 2008. ROS and p53: a versatile partnership. Free Radic Biol Med. 44:1529–1535.
Lluís, L., Taltavull, N., Muñoz-Cortés, M., Sánchez-Martos, V., Romeu, M., Giralt, M., Molinar-Toribio, E., Torres, JL., Pérez-Jiménez, J., Pazos, M., Méndez, L., Gallardo, JM., Medina, I. & Nogués, MR. 2013. Protective effect of the omega-3 polyunsaturated fatty acids: eicosapentaenoic acid/docosahexaenoic acid 1.1 ratio on cardiovascular disease risk markers in rats. Lipids Health Dis. 12:140 pp.
Lu, C., Xu, W., Zhang, F., Jin, H., Chen, Q., Chen, L., Shao, J., Wu, L., Lu, Y. & Zheng, S. 2015. Ligustrazine prevents alcohol-induced liver injury by attenuating hepatic steatosis and oxidative stress. Int Immunopharmacol. 29:613–621.
Macan, M., Vukšić, A., Žunec, S., Konjevoda, P., Lovrić, J., Kelava, M., Štambuk, N., Vrkić, N. & Bradamante, V. 2015. Effects of simvastatin on malondialdehyde level and esterase activity in plasma and tissue of normolipidemic rats. Pharmacol Rep. 67:907–913.
Matsumoto, A., Thompson, D., Chen, Y., Vasiliou, V., Kawamoto, T. & Ichiba, M. 2016. Heme oxygenase 1 protects ethanol-administered liver tissue in Aldh2 knockout mice. Alcohol. 52:49–54.
Ohkawa, H., Ohishi, N. & Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
Parrish, CC. 2009. Essential fatty acids in aquatic food webs. In : Arts MT, Brett MT, Kainz MJ, editors Lipids in Aquatic Ecosystems. Springer, New York, NY, 309–326.
Peng, H-C., Chen, Y-L., Yang, S-Y., Ho, P-Y., Yang, S-S., Hu, J-T. & Yang, S-C. 2013. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats. Hepatobiliary Surg Nutr. 2:132–141.
Plat, J., Hendrikx, T., Bieghs, V., Jeurissen, MLJ., Walenbergh, SMA., van Gorp, PJ., De Smet, E., Konings, M., Vreugdenhil, ACE., Guichot, YD., Rensen, SS., Buurman, WA., Greve, JWM., Lütjohann, D., Mensink, RP. & Shiri-Sverdlov, R. 2014. Protective role of plant sterol and stanol esters in liver inflammation: insights from mice and humans. PLoS ONE. 9:e110758 pp.
Reitman, S. & Frankel, S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63.
Ren, HX., Huang, XJ., Kim, JH., Choi, Y-K. & Gu, N. 2009. Pt/Au bimetallic hierarchical structure with micro/nano-array via photolithography and electrochemical synthesis: from design to GOT and GPT biosensors. Talanta. 78:1371–1377.
Ruxton, CHS., Reed, SC., Simpson, MJA. & Millington, KJ. 2004. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet. 17:449–459.
Sánchez-Machado, DI., López-Hernández, J., Paseiro-Losada, P. & López-Cervantes, J. 2004. An HPLC method for the quantification of sterols in edible seaweeds. Biomed Chromatogr. 18:183–190.
Santos Rocha, SW., Silva, BS., Gomes, FOS., Soares e Silva, AK., Raposo, C., Barbosa, KPS., Torres, DOC., dos Santos, ACO. & Peixoto, CA. 2012. Effect of diethylcarbamazine on chronic hepatic inflammation induced by alcohol in C57BL/6 mice. Eur J Pharmacol. 689:194–203.
Shanura Fernando, IP., Asanka Sanjeewa, KK., Samarakoon, KW., Lee, WW., Kim, H-S., Kang, N., Ranasinghe, P., Lee, H-S. & Jeon, Y-J. 2017. A fucoidan fraction purified from Chnoospora minima: a potential inhibitor of LPS-induced inflammatory responses. Int J Biol Macromol. 104:1185–1193.
Shin, D-M., An, S-R., In, S-K. & Koo, J-G. 2013. Seasonal variation in the dietary fiber, amino acid and fatty acid contents of Porphyra yezoensis
. Korean J Fish Aquat Sci. 46:337–342.
Suresh, V. & Asha, VV. 2008. Preventive effect of ethanol extract of Phyllanthus rheedii Wight. On D-galactosamine induced hepatic damage in Wistar rats. J Ethnopharmacol. 116:447–453.
Uauy, R. & Valenzuela, A. 2000. Marine oils: the health benefits of n-3 fatty acids. Nutrition. 16:680–684.
Vardi, N., Parlakpinar, H., Cetin, A., Erdogan, A. & Ozturk, IC. 2010. Protective effect of β-carotene on methotrexate-induced oxidative liver damage. Toxicol Pathol. 38:592–597.
Yokoyama, M., Origasa, H., Matsuzaki, M., Matsuzawa, Y., Saito, Y., Ishikawa, Y., Oikawa, S., Sasaki, J., Hishida, H., Itakura, H., Kita, T., Kitabatake, A., Nakaya, N., Sakata, T., Shimada, K. & Shirato, K. Japan EPA lipid intervention study (JELIS) Investigators. 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 369:1090–1098.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||