Reduction in CO2 uptake rates of red tide dinoflagellates due to mixotrophy
Article information
Abstract
We investigated a possible reduction in CO2 uptake rate by phototrophic red tide dinoflagellates arising from mixotrophy. We measured the daily ingestion rates of Prorocentrum minimum by Prorocentrum micans over 5 days in 10 L experimental bottles, and the uptake rates of total dissolved inorganic carbon (CT) by a mixture of P. micans and P. minimum (mixotrophic growth), and for the predator P. micans (phototrophic growth; control) and prey P. minimum (phototrophic growth; control) alone. To account for the effect of pH on the phototrophic growth rates of P. micans and P. minimum, measurements of CT and pH in the predator and prey control bottles were continued until the pH reached the same level (pH 9.5) as that in the experimental bottles on the final day of incubation. The measured total CT uptake rate by the mixture of P. micans and P. minimum changed from 123 to 161 μmol CT kg−1 d−1 over the course of the experiment, and was lower than the CT uptake rates shown by P. micans and P. minimum in the predator and prey control bottles, respectively, which changed from 132 to 176 μmol CT kg−1 d−1 over the course of the experiment. The reduction in total CT uptake rate arising from the mixotrophy of P. micans was 7–31% of the daily CT uptake rate seen during photosynthesis. The results suggest that red tide dinoflagellates take up less CT during mixotrophy.
INTRODUCTION
Since the industrial revolution, ever increasing quantities of CO2 have been released into the atmosphere because of the burning of fossil fuels, land use changes, and cement production. During this period approximately half the CO2 has remained in the atmosphere (Keeling and Whorf 2000, Houghton et al. 2001); the ocean and land biospheres have taken up the remainder. The global oceanic sink of fossil fuel CO2 has been estimated to be 118 ± 19 petagrams of carbon, accounting for 30% of total emissions during the period 1800–1994 (Sabine et al. 1999, 2002, Lee et al. 2003). However, it is not clear whether the oceanic sink of CO2 is stable, or will vary in response to future ocean changes.
In marine environments phototrophic organisms convert CO2 to glucose via photosynthesis; part of this fixed carbon is released back into seawater during respiration by the phototrophic organisms and/or their grazers. Through these processes marine organisms influence surface CO2 concentrations, and thereby contribute to the oceanic sink of CO2 (e.g., Emerson et al. 1997, Laws et al. 2000, Lee 2001). Accordingly, a mechanistic understanding of the key processes that control CO2 uptake and release by marine algal groups will increase knowledge of the roles of marine organisms in influencing surface water CO2 concentrations in time scales ranging from days to seasons. There have been many studies on CO2 uptake and release by cyanophytes, diatoms, microflagellates, macroalgae, and symbiotic dinoflagellates (e.g., Burkhardt et al. 2001, Miyachi et al. 2003, Rost et al. 2003, Kim et al. 2015a). Although phototrophic dinoflagellates are ubiquitous and sometimes dominant in terms of the biomass of phototrophic organisms, few studies have investigated their CO2 uptake and release (Nimer et al. 1999, Giordano et al. 2005, Rost et al. 2006).
Many phototrophic dinoflagellates originally thought to be exclusively autotrophic are now known to be mixotrophic (i.e., capable of both photosynthesis and ingestion of prey) (Jacobson and Anderson 1996, Stoecker 1999, Berge et al. 2008, Burkholder et al. 2008, Kim et al. 2015b, Lee et al. 2016). Furthermore, several newly described phototrophic dinoflagellates have been revealed to be mixotrophic (Lee et al. 2014a, 2014b, Reñé et al. 2014, Lim et al. 2015). Mixotrophic dinoflagellates are able to feed on diverse prey including heterotrophic bacteria (Nygaard and Tobiesen 1993, Seong et al. 2006, Jeong et al. 2012), cyanobacteria (Jeong et al. 2005a, 2012, Glibert et al. 2009), diatoms (Yoo et al. 2009), phytoflagellates (Jeong et al. 2005b), other phototrophic dinoflagellates (Legrand et al. 1998), heterotrophic dinoflagellates (Jeong et al. 1997), and ciliates (Bockstahler and Coats 1993, Park et al. 2006). If the heterotrophic activity of mixotrophic dinoflagellates exceeds their autotrophic activity, the release of CO2 by these organisms may be greater than CO2 uptake. Therefore, feeding by mixotrophic dinoflagellates on co-occurring prey may play an important role in the CO2 cycle. There may be complex predator-prey relationships among mixotrophic dinoflagellates, as these organisms commonly co-occur in natural environments (e.g., Jeong et al. 2005b, 2010, 2015). Populations of mixotrophic dinoflagellates feeding on each other may affect the CO2 cycle because predation feeding may reduce photosynthesis or increase respiration.
Two important questions arise concerning predatory interactions amongst mixotrophic dinoflagellates: 1) do they affect seawater CO2 concentrations and 2) if so, to what degree are the concentrations affected? To answer these questions, we established one set of experimental bottles containing a mixture of the mixotrophic dinoflagellate predator Prorocentrum micans and its mixotrophic dinoflagellate prey Prorocentrum minimum, and sets of control bottles containing P. micans and P. minimum alone. Over a 5 d incubation period we measured the daily ingestion rate of P. minimum by P. micans in the experimental culture, and the rates of uptake of total dissolved inorganic carbon (CT = [CO2aq] + [HCO3−] + [CO32−]) in both the experimental culture and the control (prey and predator alone) bottles. The results of the study provide insights into the effects of phototrophic red tide dinoflagellate mixotrophy on seawater CO2 concentrations in marine ecosystems.
MATERIALS AND METHODS
Experimental organisms
P. micans PMCJH99, isolated from Jinhae Bay in 1999 and P. minimum PMJH00, isolated from Jinhae Bay in 2000 were grown at 20°C in enriched f/2 seawater media (Guillard and Ryther 1962) without silicate, under a 14 : 10 h light-dark cycle, using cool white fluorescent light (50 μmol photons m−2 s−1).
Transmission electron microscopy
Transmission electron microscopy (TEM) analysis was used to confirm predation by P. micans on P. minimum (Jeong et al. 2005b). We incubated predator and prey cells in a 250-mL polycarbonate (PC) bottle for 24 h. A 50 mL aliquot from the PC bottle was transferred to a 50-mL centrifuge tube, and the cells were fixed for 1.5 to 2 h by the addition of glutaraldehyde (final concentration 2.5%) in culture medium. Cells were centrifuged and the pellet was agarized. After several rinses with culture medium the cells were postfixed in 1% (w/v) osmium tetroxide in deionized water, then dehydrated using a graded ethanol series (50, 60, 70, 80, 90, and 100% [all v/v] ethanol, followed by two washes with 100% ethanol). The cells were embedded in Spurr’s low viscosity resin (Spurr 1969), sectioned using a RMC MT-XL ultramicrotome (Boeckeler Instruments Inc., Tucson, AZ, USA), and post-stained with 3% (w/v) aqueous uranyl acetate followed by lead citrate. Stained sections were viewed with a JEOL-1010 electron microscope (Jeol Ltd., Tokyo, Japan).
Ingestion rates
We measured the ingestion and clearance rates of P. minimum by P. micans as a function of incubation time. Dense photosynthetic cultures of P. micans and P. minimum were grown for approximately 1 month and transferred to 10 L PC bottles. 1 mL aliquots from the PC bottles were removed at various times, and cell counts were made using a compound microscope. The experimental starting concentrations of P. micans and P. minimum were established by addition of appropriate volumes of dense cultures. We established triplicate cultures for each of 1) an experimental treatment comprising a mixture of P. micans and P. minimum, 2) a control comprising the predator (P. micans) alone, and 3) a control comprising the prey (P. minimum) alone. In addition, we established an additional bottle containing a filtrate of the predator and prey culture (seawater control).
We used mixed filtrates of the two organisms as a basal medium to ensure consistent water conditions across treatment and controls. To achieve this a predator culture was filtered through a 0.7 μm GF/F filter, and volumes of this filtrate (equal to the volume of predator culture added into the predator control and experimental bottles for each predator-prey combination) were added into the prey control and seawater control bottles. A prey culture was also filtered through a 0.7 μm GF/F filter, and volumes of this filtrate (equal to the volume of prey culture added into the prey control and experimental bottles) were added into both the predator control and seawater control bottles. Two liters of f/2 medium were added to all bottles, which were then filled to 6 L with freshly filtered seawater. Each bottle was then fitted with a cap through which three silicon tubes were inserted. To determine the cell densities (cells mL−1) of predator and prey at the beginning of the experiment and after 1–5 d of incubation, a 20 mL aliquot was removed from each bottle through one of the tubes at each sampling time; 10 mL was fixed with 5% (v/v) Lugol’s solution and 10 mL was fixed with 4% (v/v) formalin. More than 300 cells in three 1 mL Sedgwick-Rafter counting chambers were enumerated. The treatment and control bottles were placed on a shelf without being refilled after subsampling, and incubated at 20°C as described above. To account for the effect of pH on ingestion rates (see next section), incubation of prey control bottles was continued until pH values attained the same pH found in the experimental bottles on the fifth day of incubation, and the prey cell density was determined daily.
For each sampling interval the predation rates of P. minimum by P. micans (cells predator−1 d−1) in the experimental bottles were calculated as described by Jeong et al. (2005c), with the exception of the calculation of growth rate (k). An increase in pH during incubation is known to affect the growth rates of P. micans and P. minimum (Hansen et al. 2007). Therefore, to obtain unique k values for P. minimum and P. micans in the experimental bottles at a given pH, we used the measured k values in the prey and predator control bottles at the same pH as in the experimental bottles.
A 30 mL aliquot was removed from each bottle at each sampling time, and the concentrations of chlorophyll a (chl-a) were measured as described by Arar and Collins (1997).
Determination of CT and pH
Seawater CT and AT for all bottles were measured using coulometric and potentiometric titration in a VINDTA system (Marianda, Kiel, Germany). The accuracy and precision of CT and AT measurements were checked daily against seawater reference materials with known CT and AT values (certified by A. Dickson, Scripps Institution of Oceanography, San Diego, CA, USA). The measurement precisions were approximately ±1.5 μmol kg−1 for both CT and AT (a total of 14 measurements for each parameter, one set of measurements per day).
Seawater pH values for all bottles were calculated from CT and AT measurements using the carbonic acid dissociation constants of Mehrbach et al. (1973) as refitted in different functional forms by Dickson and Millero (1987). This set of thermodynamic constants has proved to be the most consistent for laboratory (Lee et al. 1996, Lueker et al. 2000, Millero et al. 2006) and field (Wanninkhof et al. 1999, Lee et al. 2000, Millero et al. 2002) measurements of carbon parameters. Given the uncertainty (±1.5 μmol kg−1) in CT and AT measurements, the predicted pH values based on CT and AT measurements were accurate to ±0.005 units. To monitor changes in seawater pH in experiments such as these it is recommended that measurements of CT and AT be performed daily, because conventional pH measurement using glass electrodes does not provide stable signals in high pH solutions (pH > 8.5).
The measurements of CT and pH were performed in parallel with measurements of the growth, ingestion, and clearance rates of P. minimum by P. micans, as described in the preceding section.
Calculation of CT uptake rate
As mixotrophic dinoflagellates can perform multiple activities (including photosynthesis, feeding, digestion, and/or respiration), we choose the CT assay over the 14C-based short-term incubation method, as the former provides a measure of the net result of multiple activities. We calculated the CT uptake rate by P. micans cells (nmol CT dinoflagellate−1 d−1) in predator control bottles at each sampling interval (1 day) by dividing the reduction in CT (nmol CT g−1 d−1 or μmol CT kg−1 d−1) by the mean predator concentration (cells mL−1). The CT uptake rate by P. minimum cells in prey control bottles was also calculated by dividing the reduction in CT by the mean prey concentration at each time interval. The mean predator (and prey) concentration at each interval was calculated following the method described by Jeong and Latz (1994).
At each sampling time the expected total CT uptake rate by the populations of P. micans and P. minimum (μmol CT kg−1 d−1) in the experimental bottles was determined by summing the calculated uptake rates of their equivalent individual populations in the predator and prey control bottles, respectively. In this calculation the CT uptake rate by the population of P. micans (nmol CT g−1 d−1, or μmol CT kg−1 d−1) in the experimental bottles at each time was obtained by multiplying the mean CT uptake rate for P. micans cells (nmol CT dinoflagellate−1 d−1) measured in the predator control bottles by the mean P. micans concentration (cells mL−1) in the experimental bottles. Similarly, the CT uptake rate by P. minimum was calculated by multiplying the mean CT uptake rate of P. minimum cells measured in the prey bottles by the mean P. minimum concentration in the experimental bottles. To minimize the effect of pH on the CT uptake rate, the mean CT uptake rates (nmol CT dinoflagellate−1 d−1) obtained from the prey and predator control bottles at the same pH as in the experimental bottles were used.
Statistical treatment of results
Experimental data were treated with linear regression analysis of variance. If the p-value is smaller than 0.05, the ANOVA test implies that a relation does exist between variables. Using this result along with the scatter plot of the figure consisting of a pair of variables (one for x-axis, and one for y-axis), it can be concluded that the relationship between two variables is linear.
RESULTS
Cell abundance
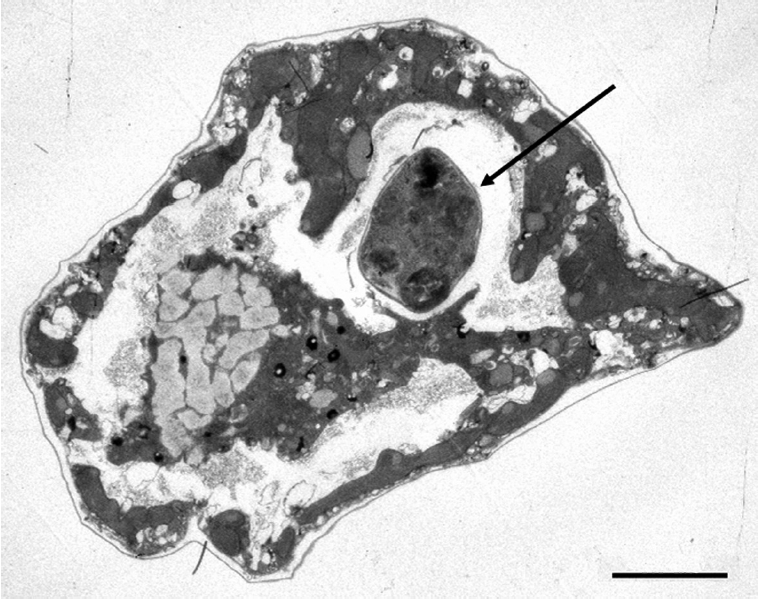
A TEM image (Fig. 1) shows a P. minimum cell within a cell of the P. micans predator. Using an inverted microscope we previously captured an image of P. micans engulfing a P. minimum cell through the sutures (Jeong et al. 2005b).
With increasing incubation time, the abundance of P. micans in the experimental treatment bottles increased from 550 cells mL−1 (day 0) to 3,596 cells mL−1 (day 5), whereas abundance in the predator control bottles increased from 562 to 4,290 cells mL−1 (Fig. 2A). Over the same period the abundance of P. minimum in the experimental treatment bottles increased from 3,820 to 16,639 cells mL−1, whereas their abundance in the prey control bottles increased from 3,878 to 30,333 cells mL−1 (Fig. 2B).
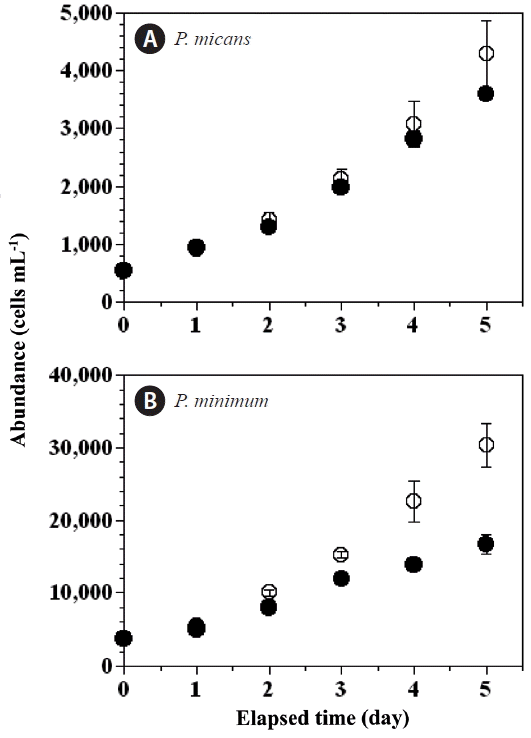
Cell abundances (cells mL−1) of the predator Prorocentrum micans (A) and the prey P. minimum (B) as a function of incubation time in the predator and prey control bottles (open circles), and the experimental treatment bottles (closed circles). Symbols and error bars represent means ± 1 standard error (n = 3).
Changes in chl-a and pH
The chl-a concentration in the predator and prey control bottles increased (day 0–5) from 14.5 to 76.9 ng mL−1, and 8.7 to 28.9 ng mL−1, respectively (Fig. 3A & B), whereas the chl-a concentration in the experimental treatment bottles increased from 20.2 to 82.4 ng mL−1 over the same period (Fig. 3C). Over the same period (day 0–5) the pH increased considerably in the predator control (from 8.36 to 9.47), the prey control (from 8.27 to 8.73), and the experimental treatment (from 8.38 to 9.57) bottles (Fig. 4A–C), whereas the pH in the seawater control bottle remained approximately unchanged (from 8.10 to 8.12).
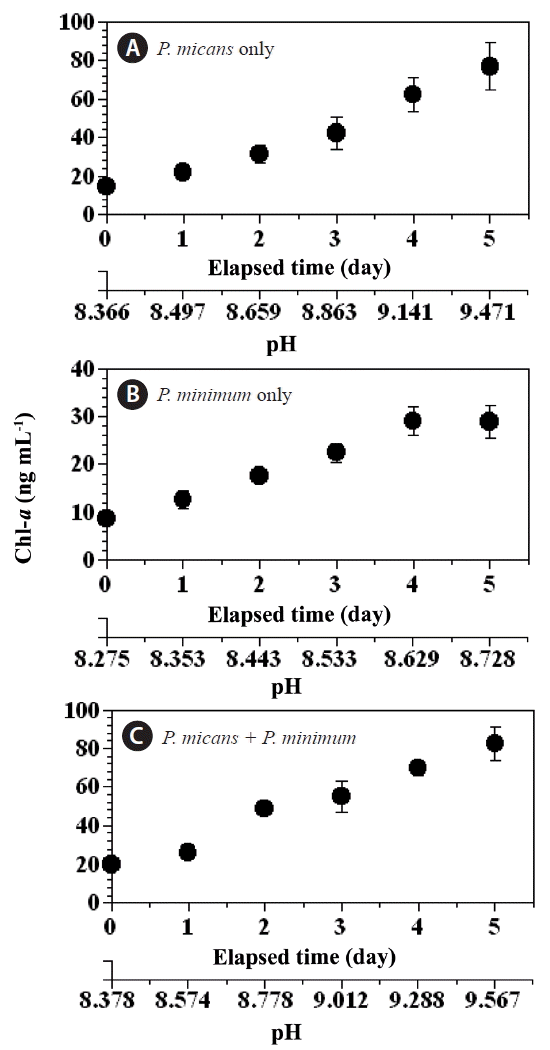
Concentrations of chlorophyll-a (chl-a, ng mL−1) in the predator Prorocentrum micans (A) and prey P. minimum (B) control bottles, and the experimental treatment bottles (C) as a function of incubation time and seawater pH. Symbols and error bars represent means ± 1 standard error (n = 3).

Seawater pH (seawater scale, kg per seawater unit) in the predator Prorocentrum micans (A) and prey P. minimum (B) control bottles, and the experimental treatment bottles (C) as a function of incubation time and total dissolved inorganic carbon concentration (CT). Symbols and error bars represent means ± 1 standard error (n = 3).
Changes in CT concentration
During the incubation period (day 0–5) the concentration of CT in the predator control and prey control bottles decreased from 1,800 to 1,100 μmol CT kg−1 and from 1,850 to 1,550 μmol CT kg−1, respectively (Fig. 5A & B). The CT value for the experimental treatment bottles decreased from 1,790 to 1,060 μmol CT kg−1 (Fig. 5C), whereas The concentration of CT in the seawater control bottle remained between 2,092 and 2,099 μmol CT kg−1.
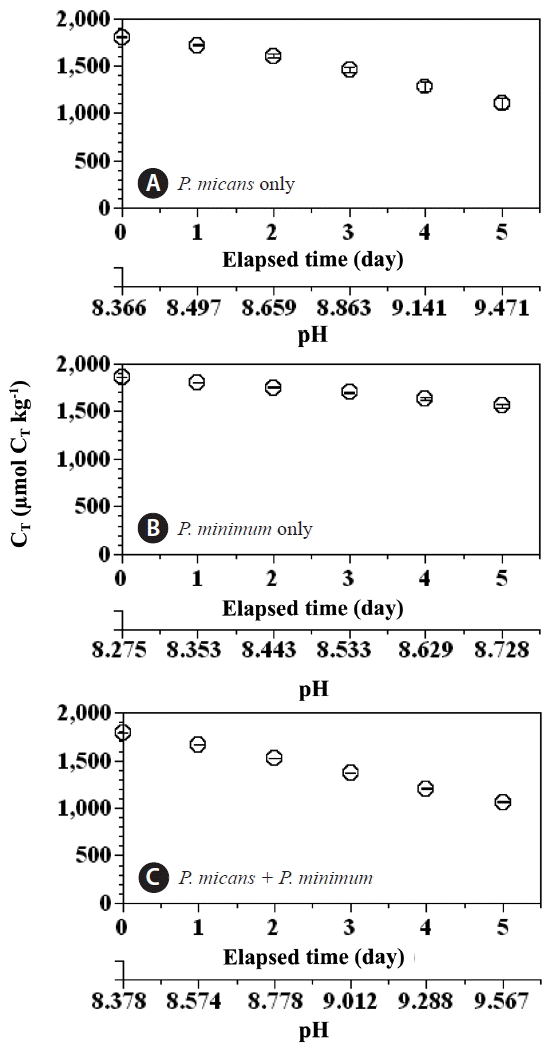
Concentrations of total dissolved inorganic carbon (CT; μmol CT kg−1) in the predator Prorocentrum micans (A) and prey P. minimum (B) control bottles, and the experimental treatment bottles (C) as a function of incubation time and seawater pH. Symbols and error bars represent means ± 1 standard error (n = 3).
The effect of pH on growth rates of Prorocentrum micans and Prorocentrum minimum
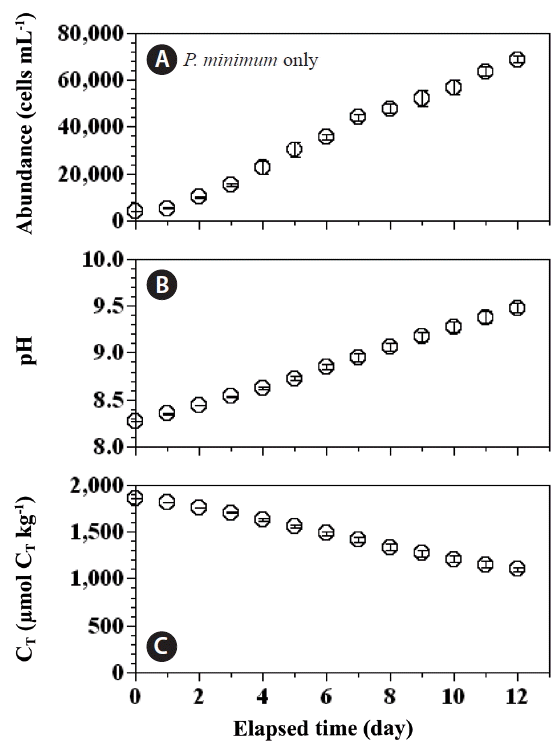
Seawater pH is known to affect the growth rates of P. micans and P. minimum; in general, the higher the pH the lower the growth rate. The pH in the predator control and experimental treatment bottles increased more rapidly than in the prey control bottles. Therefore, our measurements of abundance of P. minimum, pH, and CT in the prey control bottles were extended up to day 12 (Fig. 6), as opposed to day 5 in the predator control and experimental bottles. The abundance of P. minimum in the prey control bottles increased from 35,708 cells mL−1 at day 6 to 68,583 cells mL−1 at day 12 (Fig. 6A). The pH increased in the prey control bottles from 8.85 to 9.48 (Fig. 6B) between day 6 and day 12, and the CT concentrations decreased from 1,480 to 1,100 μmol CT kg−1 (Fig. 6C).
Rate of carbon (CT) uptake
The CT uptake rates by P. micans in the predator control bottles (0.049–0.118 nmol CT dinoflagellate−1 d−1) (Fig. 7A) were an order of magnitude higher than those for P. minimum in the prey control bottles (0.004–0.011 nmol CT dinoflagellate−1 d−1) (Fig. 7B). However, the chl-a specific CT uptake rates of P. micans in the predator control bottles (112–220 μmol CT [mg chl-a] −1 h−1) were similar in magnitude to those of P. minimum in the prey control bottles (98–254 μmol CT [mg chl-a] −1 h−1). The CT uptake rates (per cell) of P. micans and P. minimum were significantly positively correlated with the CT concentration (p < 0.05, linear regression ANOVA) (Fig. 7).
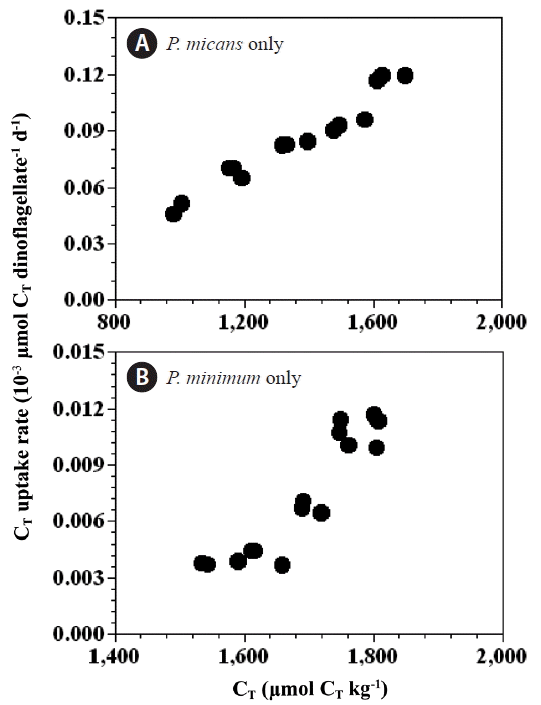
Rate of CT uptake (10−3 μmol CT dinoflagellates−1 d−1) by Prorocentrum micans (A) and P. minimum (B) as a function of CT. Symbols and error bars represent means ± 1 standard error (n = 3).
The total CT uptake rate of the combined populations of P. micans and P. minimum in the experimental bottles was 123–161 μmol CT kg−1 d−1, which was lower than the expected total CT uptake rate (136–212 μmol CT kg−1 d−1) solely by a phototrophic growth of P. micans and P. minimum (Fig. 8A). With increasing incubation time the difference in the total CT uptake rate (expected total CT uptake rate - measured total CT uptake rate) increased from 8 to 56 μmol CT kg−1 d−1 (Fig. 8B). Even for CT uptake corrected for the effect of pH, the measured total CT uptake rate was lower than the expected rate (132–176 μmol CT kg−1 d−1) (Fig. 8C), and the reduction in the total CT uptake rate arising from the mixotrophy of P. micans increased from 6 to 25 μmol CT kg−1 d−1 (Fig. 8D).

Expected (open circles) and measured (closed circles) total CT uptake rates (A & C) by the red tide dinoflagellates and their anomalies (B & D) (dCT uptake rate = expected total CT uptake rate - measured total CT uptake rate) as a function of the incubation time, seawater pH, and total dissolved inorganic carbon concentration (CT). In A and B the total CT uptake rates and corresponding anomalies were not corrected for the effect of pH, whereas in C and D the total CT uptake rates and corresponding anomalies were corrected for pH. Symbols and error bars represent means ± 1 standard error (n = 3).
Ingestion rates
The higher pH (or lower CT) observed in the prey control bottles between day 6 and day 12 could have lowered the growth rate of P. minimum. Thus, we calculated two ingestion rates of P. minimum by P. micans; one rate corrected for pH effects and the other not so corrected. To account for the effect of pH on ingestion rate, we calculated the ingestion rate of P. minimum by P. micans by replacing the growth rate of P. minimum in the experimental bottles by that obtained for P. minimum in the prey control bottles at similar pH and CT levels.
Without correcting for pH effects the apparent predation rate of P. minimum by P. micans was 236–2,784 cells d−1 at day 5 (Fig. 9A). In contrast, the pH-corrected predation rate of P. minimum by P. micans was 630–1,946 cells d−1 at day 5 (Fig. 9B).
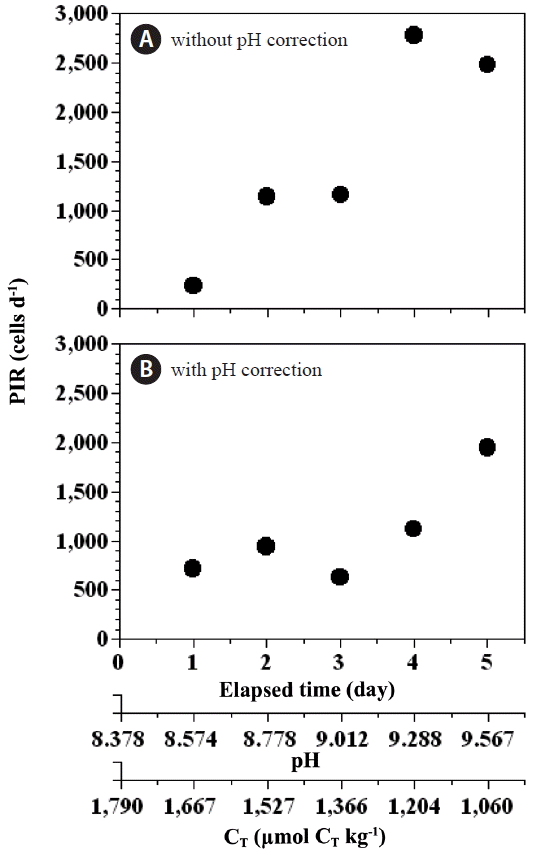
Total Prorocentrum minimum cells ingested by P. micans per day (PIR, cells d−1) as a function of incubation time, seawater pH, and total dissolved inorganic carbon concentration (CT) without correction for the effect of pH (A), and with correction for the effect of pH (B). Symbols and error bars represent means ± 1 standard error (n = 3).
Correlations
Our results indicate that the differences between the expected and measured rates of CT uptake in the experimental treatment bottles were significantly positively correlated with the rate of predation of P. minimum by P. micans (p < 0.05 for both, linear regression ANOVA) (Fig. 10A & B). These relationships suggest that feeding by P. micans on P. minimum may be an important factor in controlling respective rates of carbon uptake.
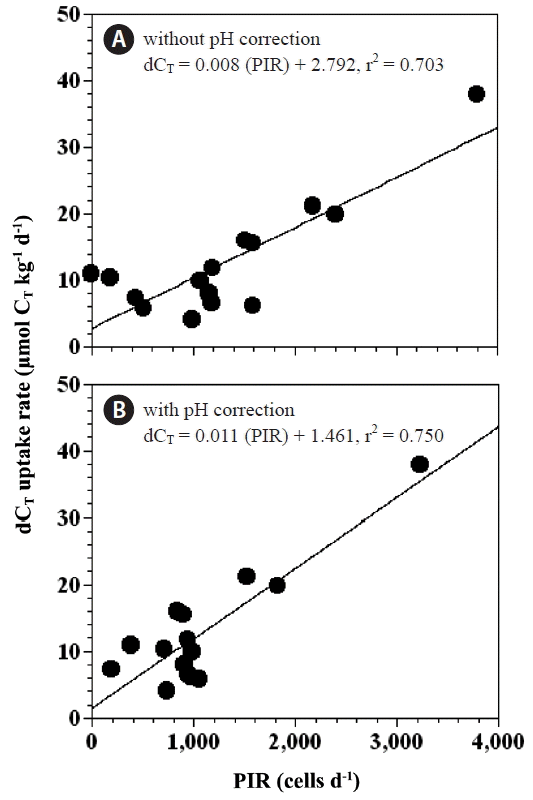
Anomalies in CT uptake (dCT uptake rate) as a function of total P. minimum cells ingested by P. micans per day (PIR, cells d−1) without correction for the effect of pH (A), and with correction for the effect of pH (B). The equations for the linear regressions are as follows: dCT uptake rate (μmol CT kg−1 d−1) = 0.008 × (PIR) + 2.792, r2 = 0.703 (p < 0.01) (A) and dCT uptake rate (μmol CT kg−1 d−1) = 0.011 × (PIR) + 1.461, r2 = 0.750 (p < 0.05) (B).
DISCUSSION
Few studies have reported CO2 uptake rates (and/or maximum chl-a specific CO2 uptake rates) for phototrophic dinoflagellates (Rost et al. 2006). In this study the CT uptake rate of P. micans (0.049–0.118 nmol CT dinoflagellate−1 d−1) was approximately 10 times higher than that of P. minimum (0.004–0.011 nmol CT dinoflagellate−1 d−1). Both the cell volume and chl-a content of P. micans are approximately 10-fold those of P. minimum. Therefore, the chl-a specific CT uptake rates of P. micans were comparable in magnitude to those of P. minimum, indicating that the concentration of chl-a is an important factor in determining CT uptake rate. The measured maximum chl-a specific CT uptake rates of P. micans (220 μmol CT [mg chl-a]−1 h−1) and P. minimum (254 μmol CT [mg chl-a]−1 h−1) in the present study are similar in magnitude to those of the diatoms Phaeodactylum tricornutum, Thalassiosira weissflogii and Skeletonema costatum, the prymnesiophyte Phaeocystis globosa (ca. 200–300 μmol [mg chl-a]−1 h−1) (Burkhardt et al. 2001, Rost et al. 2003), and the coccolithophorid Emiliania huxleyi (ca. 200–300 μmol [mg chl-a]−1 h−1) (Rost et al. 2003). The CT uptake rates, normalized to the chl-a content, are similar in magnitude across planktonic groups. Therefore, the chl-a concentrations in marine algae may be useful in estimating CT uptake rates.
The maximum chl-a specific CT uptake rate of P. minimum calculated in the present study is much lower than the maximum chl-a specific HCO3− uptake rate (ca. 700 μmol [mg chl-a]−1 h−1) determined by Rost et al. (2006). The latter uptake rate for P. minimum was measured using the 14C method, in which HCO3− is taken up over a short period. This evidence suggests that the maximum chl-a specific HCO3− uptake rate obtained using the 14C method is higher than that measured using relatively long-term incubation involving a light-dark cycle.
The mean CT uptake rates per cell (P. micans or P. minimum) were positively correlated with CT concentrations, indicating that this factor also affects the C uptake rate, as previously reported for diatoms and the mixotrophic dinoflagellate Heterocapsa triquetra (Burkhardt et al. 2001, Rost et al. 2003, 2006).
The results of the present study show that feeding by a mixotrophic dinoflagellate predator on a phototrophic dinoflagellate prey can lead to a substantial reduction in total CT uptake rates. The degree of reduction in CT uptake rates because of the mixotrophy of P. micans was 7–31% of the daily CT uptake rates during photosynthesis. The reductions in CT uptake rate from mixotrophy were positively correlated with the ingestion rates of P. minimum by P. micans (Fig. 10). Two possibilities could account for this trend. The first is that P. micans may photosynthesize less (i.e., the photosynthetic rate may be considerably reduced) during feeding on P. minimum and associated digestion. Second, the respiration rate of P. micans in the mixotrophic mode was greater than in the autotrophic mode, suggesting that more organic carbon may be converted to CO2 in the mixotrophic mode. The present study thus indicates that reduction in CT uptake by mixotrophic dinoflagellates feeding on co-occurring prey should be taken into account in ecosystem models describing CO2 dynamics.
When considering ingestion rates among different dinoflagellate predators feeding on the same prey species (e.g., Jeong et al. 2005b, 2010, 2015), it should be noted that the degree of reduction in the rate of CT uptake arising from mixotrophy may be different from that of P. micans. To better understand the dynamics of the CO2 cycle in a given ecosystem, predator-prey relationships among mixotrophic dinoflagellates and co-occurring plankton should be investigated, as should the ingestion rates of mixotrophic dinoflagellates on co-occurring plankton prey. The carbon dynamics within a given ecosystem can be reasonably described by measuring in situ ingestion rates of dominant mixotrophic dinoflagellates on co-occurring algal prey. Many mixotrophic dinoflagellates are known to feed on diverse phytoplankton including cyanobacteria, haptophytes, cryptophytes, raphidophytes, and other mixotrophic dinoflagellates (Skovgaard et al. 2000, Jeong et al. 2005a, Lee et al. 2015). Therefore, feeding by mixotrophic dinoflagellates on prey is likely to occur frequently because predator and prey usually coexist in natural environments. The CO2 uptake by mixotrophic dinoflagellates and co-occurring microalgae is likely to be lower when dinoflagellate phagotrophy is occurring than when phagotrophy is absent or rare.
Despite the low availability of CO2 in the external environment and the low affinity of algal RUBISCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) for CO2 (Badger et al. 1998), most algae (including coccoliths and diatoms), and cyanobacteria can actively perform photosynthesis by utilizing either CO2 or HCO3− (or both) as external sources of inorganic carbon via a CO2 concentrating mechanism (CCM) (Reinfelder 2011). Little is known about the mechanisms of CT uptake in dinoflagellates; however, several species have a CCM in that they are known to have the capability to accumulate inorganic carbon during photosynthesis (Dason et al. 2004). P. micans is reported to have a 10-fold higher CT concentration than seen in the external environment (Nimer et al. 1999). P. micans may not need a CCM when feeding on prey, instead reserving CT for later use when the population of prey is low. Acquiring and reserving CT via phagotrophy may provide dinoflagellates with a competitive advantage over strictly photosynthetic diatoms. Mixotrophy in dinoflagellates is a unique survival strategy at low CO2 levels in modern oceans. Dinoflagellates are known to have occurred in the oceans ~400 million years ago (the early Devonian), when CO2 levels (approximately 3,040 μatm) were eight times higher than at present (~402 μatm) (Falkowski and Raven 1997). As the CO2 concentration decreased from the early Devonian to the present, dinoflagellates may have risen in importance because of their unique mixotrophic survival strategy. It is worth exploring the relative importance of autotrophy and mixotrophy in dinoflagellates in response to long-term changes in CO2 concentration.
ACKNOWLEDGEMENTS
This work is supported by Korea Ministry of Environment as “The GAIA project” (2014000540010).