Taxonomic revision of the genus Herposiphonia (Rhodomelaceae, Rhodophyta) from Korea, with the description of three new species
Article information
Abstract
We examined the species diversity of Herposiphonia on Korean coasts, based on a combination of morphology and molecular analyses of the mitochondrial COI-5P DNA barcode marker and plastid rbcL gene. We report the presence of eight species including three novel species: H. donghaensis sp. nov., H. jejuinsula sp. nov., H. sparsa sp. nov., H. caespitosa, H. fissidentoides, H. insidiosa, H. parca, and H. subdisticha. Specimens were separated into eight clades in both the COI-5P and rbcL gene analyses, with 1.3–19.6 and 6.6–15% interspecific sequence divergence, respectively. These eight species are also distinguishable by several morphological characteristics such as: branching pattern (d/i pattern in H. donghaensis sp. nov. and H. sparsa sp. nov.; d/d/d/i pattern in others), shape of determinate branch (ligulate in H. fissidentoides; terete in others), number of vegetative trichoblasts (1–2 in H. insidiosa and H. sparsa sp. nov.; 3–4 in H. caespitosa; absent in others), and number of segments and pericentral cells in determinate branches. About three novel species revealed by our analyses, H. donghaensis sp. nov. is newly discovered, and H. jejuinsula sp. nov. and H. sparsa sp. nov. were previously reported in Korea as H. nuda and H. secunda, respectively. Our results show that DNA barcoding and rbcL analyses are useful for delimiting species boundaries and discovering cryptic species diversity in the genus Herposiphonia.
INTRODUCTION
First established by Nägeli (1846), the red algal genus Herposiphonia currently includes 56 species found in tropical to warm-temperate regions of the world (Guiry and Guiry 2017). The genus is characterized by a dorsiventral habit, polysiphonous and uncorticated thalli, with a more or less regular sequence of exogenous determinate and indeterminate branches (Hollenberg 1968). Apices of main axis are usually inrolled upwardly, and vegetative trichoblasts and reproductive structures are formed exclusively on determinate branches (Masuda et al. 2006).
Taxonomic studies of Herposiphonia have primarily utilized morphological features and are mostly regional. For instance, in Korea, eight species of Herposiphonia: H. caespitosa Tseng, H. fissidentoides (Holmes) Okamura, H. insidiosa (Greville ex J. Agardh) Falkenberg, H. nuda Hollenberg, H. parca Setchell, H. plumula (J. Agardh) Falkenberg, H. secunda (C. Agardh) Ambronn and H. subdisticha Okamura have been reported to occur in intertidal to subtidal habitats (Lee et al. 1992, Lee 2008). Other regional morphological studies have documented species of Herposiphonia from Brazil (Silva and Fujii 2012), China (Ding et al. 2016), Hawaii (Hollenberg 1968), Hong Kong (Tseng 1943), Japan (Masuda et al. 2006), South Africa (Wynne 1984), and Venezuela (García et al. 2008). The diversity of Herposiphonia, however, has not been studied using molecular analyses, despite the large number of species.
Recent studies of the family Rhodomelaceae used the plastid-encoded ribulose-1,5-bisphosphate carboxylase / oxygenase large subunit gene (rbcL) and uncovered several novel species (Kim et al. 2012, Kang and Kim 2013). DNA barcoding, such as the 5′ end of mitochondrial-encoded cytochrome c oxidase subunit I gene (COI-5P) has also proved the effective for species-level identification of red algae (Le Gall and Saunders 2010, Saunders and McDonald 2010, Sherwood et al. 2010, Yang and Kim 2015). The COI-5P marker has been successfully utilized in studies of the genus Herposiphonia and revealed cryptic species diversity in the Iberian Peninsula and Western Australia (Díaz-Tapia and Bárbara 2013, Huisman et al. 2015). The barcoding gap observed using the COI-5P marker allows specimens to be assigned to their genetic species unambiguously (Le Gall and Saunders 2010).
In the present study, we aimed to investigate the species diversity of Korean Herposiphonia based on morphology and molecular analyses. We utilized DNA barcoding using COI-5P marker to identify Herposiphonia species groups, and inferred their phylogenetic relationships using the plastid rbcL gene. Our results demonstrate the presence of eight species of the genus Herposiphonia from Korea including three distinct new species.
MATERIALS AND METHODS
Samples of Herposiphonia were collected from the intertidal and subtidal zones in Jeju Island and the east coast of Korea (Supplementary Table S1). Samples were assigned voucher numbers and permanent slide vouchers were deposited in the Herbarium of Jeju National University (JNUB) and National Institute of Biological Resources (KB), Korea. Samples for morphological study were preserved in 3–5% formalin / seawater and sectioned by using a freezing microtome NK-101-II (Nippon Optical Works Co. Ltd., Tokyo, Japan) or by hand. Photographs were taken with a digital camera (Canon EOS 600D; Canon, Tokyo, Japan) mounted on a microscope (Olympus BX43; Olympus, Tokyo, Japan). All images were imported into the Adobe PhotoShop CS6 software (Adobe Systems Inc., San Jose, CA, USA) for plate assembly.
Parts of samples dried in silica gel were used for DNA extraction. DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions except for using half of the elution buffer at the elution step. The primer pairs used for polymerase chain reaction (PCR) amplification and sequencing reaction of rbcL gene were rbcLJNF1-rbcLJNR1 and rbcLJNF2-rbcLJNR2 (Kang and Kim 2013). The primer pairs used for COI-5P region followed Kim et al. (2010). Amplification conditions for both rbcL and COI-5P were 7 min at 97°C for denaturation, followed by 35 or 37 cycles of 1 min at 97°C, 1 min at either 45°C or 47°C, and 2 min at 72°C, with final 7 min extension cycle at 72°C and a soak cycle at 4°C. PCR products were purified using the AccuPrep PCR Purification Kit (Bioneer, Daejeon, Korea) following the manufacturer’s instructions. Nucleotide sequences of rbcL and COI-5P region were determined on both strands of PCR amplification products at the Macrogen sequencing facility (Macrogen Inc., Seoul, Korea). Electropherogram outputs from each sample were edited using Chromas ver. 1.45 software (Chromas, Queensland, Australia). Total rbcL and COI-5P sequences were organized using the multiple-sequence editing program MEGA ver. 6.05 (Tamura et al. 2013) and aligned visually (Supplementary Table S1).
DNA barcode data analysis was conducted to define species boundaries of the genus Herposiphonia using MEGA ver. 6.05 (Tamura et al. 2013) with distances collected under a Kimura 2-parameter model, and neighbor-joining (NJ) was used to provide a visual display of COI-5P variation within and between species. Maximum likelihood (ML) analysis was conducted to estimate the phylogenetic relationships of the genus Herposiphonia using RAxML software (Stamatakis 2006) under the GTR + I + Γ evolutionary model. We used 1,000 independent tree inferences using -# option (1,000 distinct ML) with the algorithm “a” using -f option (rapid bootstrap analysis) to search the best-scoring tree in one program run.
RESULTS AND DISCUSSION
Molecular analyses
We analyzed 32 COI-5P sequences of Korean Herposiphonia specimens together with 29 sequences from GenBank (Supplementary Table S1). These sequences consisted of 613 nucleotides with no insertion or deletion mutations, permitting unambiguous alignment of all sequences. Of these nucleotides, 235 positions (38.33%) were variable and 208 positions (33.93%) were parsimoniously informative. An unrooted phylogram using NJ analysis showed that 32 Korean Herposiphonia specimens were resolved into eight species groups that could be assigned to five known species, H. caespitosa, H. fissidentoides, H. insidiosa, H. parca, and H. subdisticha, and three novel species, H. donghaensis sp. nov., H. jejuinsula sp. nov., H. sparsa sp. nov., based on the combination of interspecific (1.3–19.6% range) and intraspecific (0–0.8% range) sequence divergence (Fig. 1). This barcoding gap is slightly conservative compared with results of similar analyses in several red algal taxa where the maximum intraspecific and minimum interspecific divergences were 0.9 vs. 9.2% in Gracilariaceae, 0.9 vs. 4.22% in Rhodymeniales, and 1.36 vs. 3.2% in Gelidiales (Freshwater et al. 2010, Kim et al. 2010, Saunders and McDonald 2010, Koh et al. 2013) but similar to that observed in Phyllophoraceae as 0.75 vs. 1% (Le Gall and Saunders 2010).
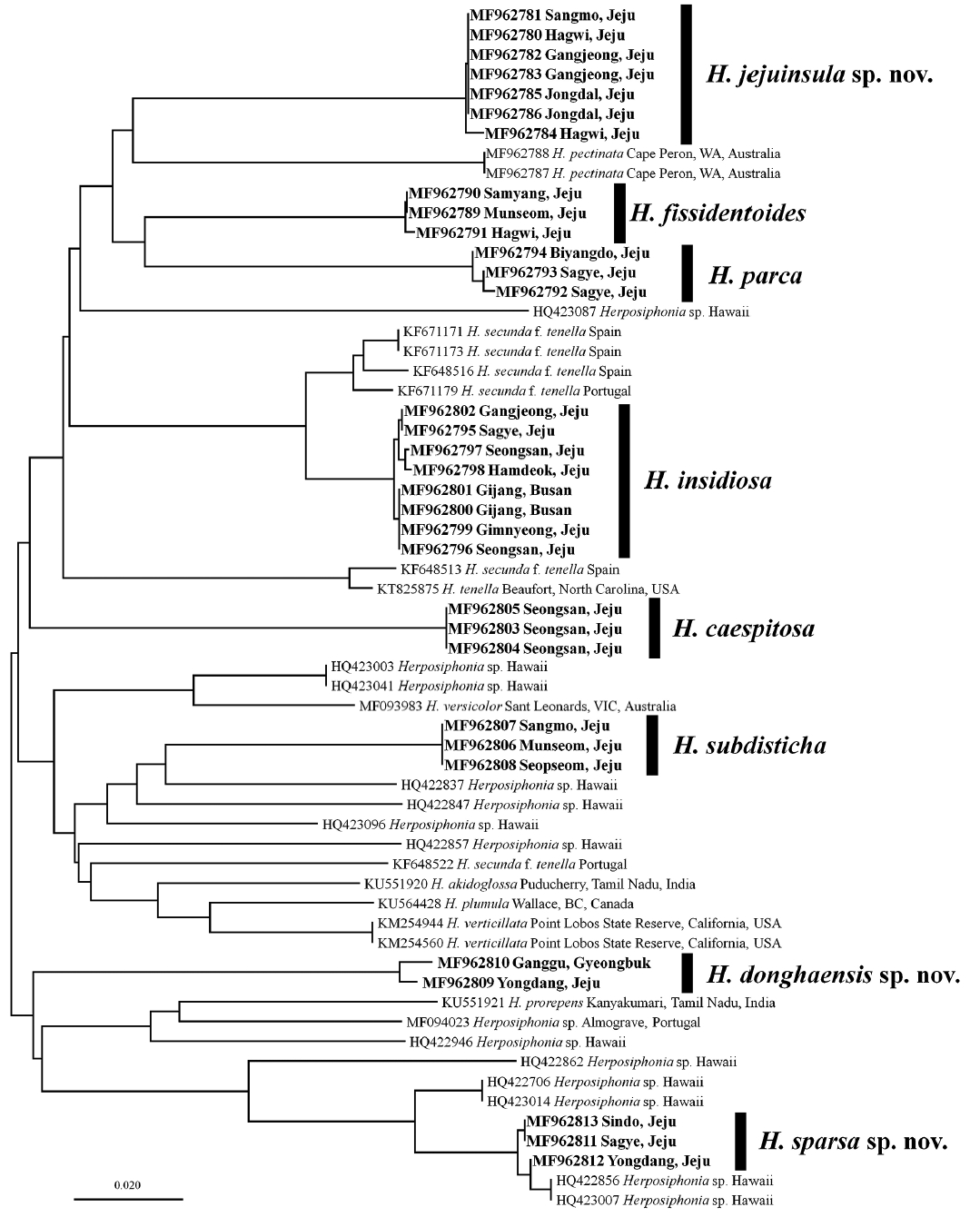
Unrooted phylogram generated using neighbor-joining analysis of COI-5P sequences of Herposiphonia specimens.
For 51 samples, 1,166 nucleotides of the rbcL gene were analyzed including three outgroups: Pterosiphonia cloiophylla (C. Agardh) Falkenberg (GQ867081); Symphyocladia marchantioides (Harvey) Falkenberg (GU731229); and Womersleyella setacea (Hollenberg) R. E. Norris (JX828160). Insertion or deletion mutations were not found. Of these, 418 positions (35.84%) were variable and 365 (31.3%) were parsimoniously informative. The sequence divergence ranged from 6.6 to 15% among Herposiphonia species. The genus Herposiphonia formed a well-defined monophyly with 100% bootstrap support in ML analyses and eight Korean Herposiphonia species were also well distinguished by 100% bootstrap support (Fig. 2). As the case of COI-5P barcoding analysis, H. donghaensis sp. nov., H. jejuinsula sp. nov., and H. sparsa sp. nov., were also resolved. Two of them, H. jejuinsula sp. nov. and H. sparsa sp. nov., had been previously recorded as H. nuda and H. secunda, respectively (Lee 2008, Nam and Kang 2012). In this analysis, H. sparsa sp. nov. formed a clade with two unidentified species from Australia (MF094072 from Queensland, MF094073 from Western Australia) (Díaz-Tapia et al. 2017) with 100% support value and was positioned next to H. donghaensis sp. nov. with moderate support value (84%). Similarly, H. subdisticha was closely related to an unidentified Herposiphonia species (MF094075) (Díaz-Tapia et al. 2017) from Western Australia with full support value and H. caespitosa was related to another unidentified Herposiphonia species (MF094071) (Díaz-Tapia et al. 2017) from Queensland, Australia with moderate support value (88%).
Morphological observations
Morphological characteristics of eight Korean Herposiphonia species identified from the molecular phylogenetic analyses are as follows.
Herposiphonia donghaensis Y. H. Koh et M. S. Kim sp. nov
Holotype
140112-99-1, vegetative thallus, Jan 12, 2014 (Fig. 3A), deposited in the Herbarium of Jeju National University, Jeju, Korea (JNUB).
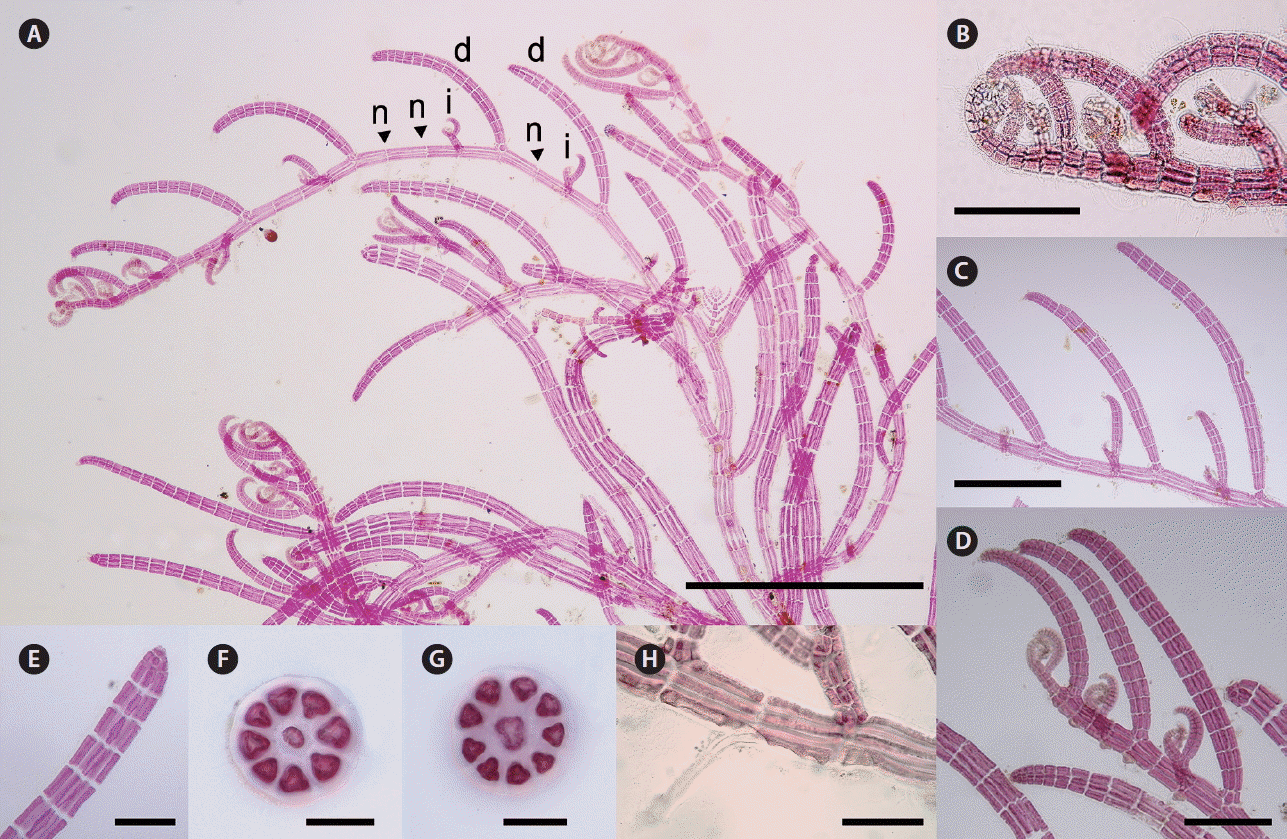
Herposiphonia donghaensis sp. nov. Y. H. Koh et M. S. Kim. (A) Vegetative thallus collected from Jukbyeon, Uljin, Gyeongbuk, Korea showing d/i branching pattern with 1 or 2 bare nodes between indeterminate branches (d, determinate branch; i, indeterminate branch; n, naked node). (B) Apical part of main axis. (C & D) Determinate branches composed of 12–16 segments. (E) Apical part of a determinate branch showing absence of vegetative trichoblast. (F) Cross section of a prostrate branch. (G) Cross section of a determinate branch. (H) A transparent rhizoid cut-off from pericentral cells. Scale bars represent: A, 1,000 μm; B & D, 200 μm; C, 400 μm; E & H, 100 μm; F & G, 40 μm.
Isotype
140112-99-2, deposited in the National Institute of Biological Resources (KB: NIBRAL0000152592).
Type locality
Jukbyeon, Uljin, Gyeongbuk, Korea (37°03′28″ N, 129°25′49″ E).
Etymology
The specific epithet (donghaensis) refers to the Korean name of East Sea.
Korean name
동해거미줄.
Molecular vouchers
MF962751-MF962754 (rbcL); MF962809-MF962810 (COI-5P).
Other specimens examined
140112-48, 140112-49 (Ganggu, Yeongdeok, Gyeongbuk, Korea; Jan 12, 2014); 140112-100 (Jukbyeon, Uljin, Gyeongbuk Korea; Jan 12, 2014).
Description
Thalli are delicate and red to dark red in color. Primary axes are prostrate, with upwardly circinate apices, attached to other algal species such as Gelidium spp. and Chondracanthus spp. by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 3H). Prostrate branches are terete, with 8–9 pericentral cells (Fig. 3C & F). They are 60–100 μm in diameter and segments are 120–150 μm in length, with length : diameter (L : D) ratio 0.7–2. Indeterminate branches (i) arise on primary prostrate axes on alternate sides from every third or fourth segment, with a determinate branch (d) and one or two naked nodes (n) on intervening segments in the dorsal position (d/i pattern) (Fig. 3A–D). Some indeterminate branches grow like main axis possessing branches but others remain short or rudimentary (Fig. 3A). Determinate branches are also terete, with 8–9 pericentral cells and are 12–16 segments long (Fig. 3C & D). They are 55–80 μm in diameter with segment L : D ratio 0.8–1 (Fig. 3G). Vegetative trichoblasts are absent (Fig. 3E).
Remarks
H. donghaensis sp. nov. was collected from the central region of the east coast of Korean Peninsula. This species had previously been misidentified as H. parca based on the rbcL sequence (JX828127) collected from the same location (Bárbara et al. 2013, not include in our phylogenetic analysis because the sequences have several ambiguous characters). We recognized as a novel species here because H. parca has a d/d/d/i branching pattern (Hollenberg 1968) in contrast to that of our species (d/i patterns) with one or two irregular naked nodes. Other species that has the d/i branching pattern includes H. arcuata Hollenberg, H. dubia Hollenberg, H. pacifica Hollenberg, H. ramosa C. K. Tseng, H. secunda, and H. variabilis Hollenberg (Hollenberg 1968, Tseng 1943, Schneider and Searles 1997) but they can be distinguished from H. donghaensis in various vegetative features such as diameter of prostrate axes (140–190 μm in H. arcuata, 180–200 μm in H. ramosa vs. 60–100 μm in H. donghaensis), number of segments in determinate branches (45–75 in H. dubia, 60–80 in H. pacifica vs. 12–16 in H. donghaensis), number of naked nodes (1–6 in H. variabilis vs. 1–2 in H. donghaensis), and number of vegetative trichoblasts (1–2 in H. secunda vs. absent in H. donghaensis) (Hollenberg 1968, García et al. 2008, Lee 2008, Xia 2011, Nam and Kang 2012, Silva and Fujii 2012). In our molecular analyses, H. donghaensis sp. nov. is clearly separated from other Herposiphonia species with sequence divergence of 14.4–18.9% in COI-5P and 10.9–15.3% in rbcL.
Herposiphonia jejuinsula Y. H. Koh et M. S. Kim sp. nov
Holotype
YHK-130522-1, female gametophyte, May 22, 2013 (Fig. 4A), deposited in the Herbarium of Jeju National University, Jeju, Korea (JNUB).
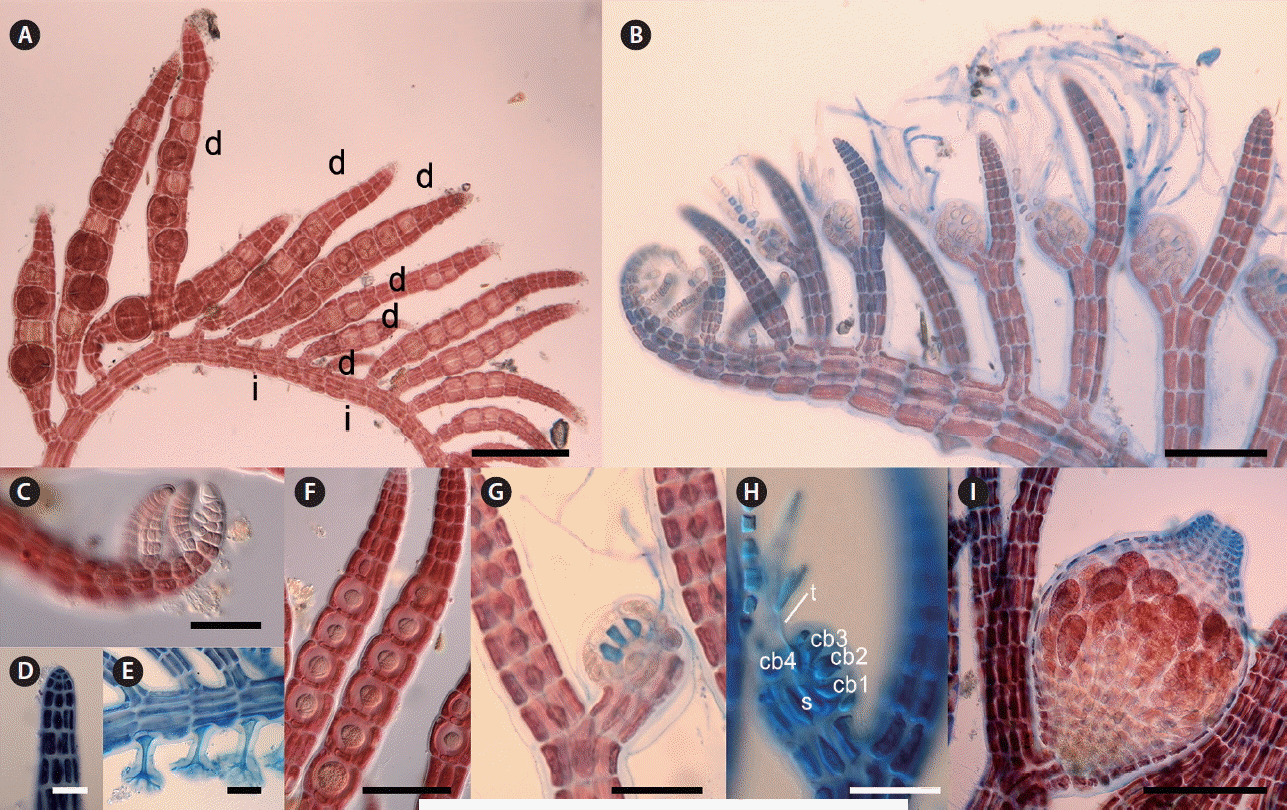
Herposiphonia jejuinsula sp. nov. Y. H. Koh et M. S. Kim. (A) Tetrasporophyte thallus showing d/d/d/i branching pattern (d, determinate branch; i, indeterminate branch). (B) Procarps developed in the middle of fertile branches. (C) Apical part of main axis. (D) Apical part of a determinate branch showing absence of vegetative trichoblast. (E) Rhizoids cut-off from pericentral cells. (F) Straight series of tetrasporangia along determinate branches. (G & H) Procarps showing 4-celled carpogonial branches developed inside (cb, carpogonial branch; s, supporting cell; t, tricogyne). (I) Fully matured cystocarp. Scale bars represent: A & B, 200 μm; C–E, G & H, 50 μm; F & I, 100 μm.
Isotype
YHK-130522-2, tetrasporophyte, deposited in National Institute of Biological Resources (KB: NIBRRD0000001693).
Type locality
Gangjeong, Jeju, Korea (33°13′51″ N, 126°29′58″ E).
Etymology
The specific epithet (jejuinsula) refers to the location of type locality, Jeju Island.
Korean name
민털거미줄.
Molecular vouchers
MF962768-MF962772 (rbcL); MF962780-MF962786 (COI-5P).
Other specimens examined
130330-2 (Sangmo, Jeju, Korea; Mar 30, 2013); YHK131020-1 (Munseom, Jeju, Korea; Oct 20, 2013); E14014 (Hagwi, Jeju, Korea; Oct 30, 2013); 140704-70 (Jongdal, Jeju, Korea; Jul 4, 2014); YHK140729-1 (Munseom, Jeju, Korea; Jul 29, 2014); YHK140813-1 (Munseom, Jeju, Korea; Aug 13, 2014).
Description
Thalli are delicate and pink to pale red in color. Primary axes are prostrate, with upwardly circinate apices, attached to coralline algae such as Amphiroa spp. by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 4E). Prostrate branches are terete, with 6–8 pericentral cells. They are 50–80 μm in diameter and segments are 120–200 μm in length, with L : D ratio 0.5–1.2 (Fig. 4A & B). Indeterminate branches (i) arise on primary prostrate axes on alternate sides from every fourth segment, with three determinate branches (d) on intervening segments in the dorsal position (d/d/d/i pattern) (Fig. 4A & B). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary (Fig. 4A & B). Determinate branches are terete with, 5–6 pericentral cells and are 18–23 segments long (Fig. 4A & B). They are 40–60 μm in diameter with segment L : D ratio 0.5–1 (Fig. 4A, D & G). Vegetative trichoblasts are absent (Fig. 4D). Trichoblasts are formed only on gametophytes and divide pseudodichotomously (Fig. 4B). A procarp consisting of a supporting cell and a 4-celled carpogonial branch (Fig. 4G & H) is enclosed in a pericarp prior to fertilization and develops into a cystocarp following fertilization. Fertile branches bearing a developing cystocarp continue to grow and form an additional 7 to 12 segments, resulting in a cystocarp in the middle of fertile branches (Fig. 4I). Mature cystocarps are urceolate and 500–550 μm in diameter. Tetrasporangia are formed from stalk cells inside 5–8 successive segments of fertile determinate branches and grow spirally to fill the segments (Fig. 4A & F). Mature tetrasporangia are tetrahedrally divided with prominent spores of 40–55 μm diameter (Fig. 4A).
Remarks
H. jejuinsula sp. nov. has been misidentified as H. nuda in Korea due to the position of cystocarp on fertile determinate branches (Lee 2008). First described by Hollenberg (1968), H. nuda is characterized by considerably long (25–30 segments) and slender determinate branches with 4–5 pericentral cells, lack of trichoblasts, and the mid-branch location of cystocarps. Most features of H. jejuinsula are in fact similar to Hawaiian and Brazilian specimens of that species (Hollenberg 1968, Silva and Fujii 2012). However, H. jejuinsula has slenderer main axes (50–80 vs. 80–105 μm) and shorter determinate branches (18–23 segments vs. 25–44) than Hawaiian H. nuda (Hollenberg 1968, Abbott 1999). In our molecular analyses, H. jejuinsula was distinctly separated from other species of Herposiphonia by a sequence divergence of 7.5% (H. fissidentoides) to 12.4% (H. subdisticha) in rbcL and 12.1% (H. parca) to 18.9% (H. donghaensis) in COI-5P. In addition, H. jejuinsula sp. nov. was clearly separated from Hawaiian Herposiphonia specimens by a 12.2–18.5% sequence divergence in COI-5P (Sherwood et al. 2010).
Herposiphonia sparsa Y. H. Koh et M. S. Kim sp. nov
Holotype
140704-68, vegetative thallus, Jul 4, 2013 (Fig. 5A), deposited in the Herbarium of Jeju National University, Jeju, Korea (JNUB).
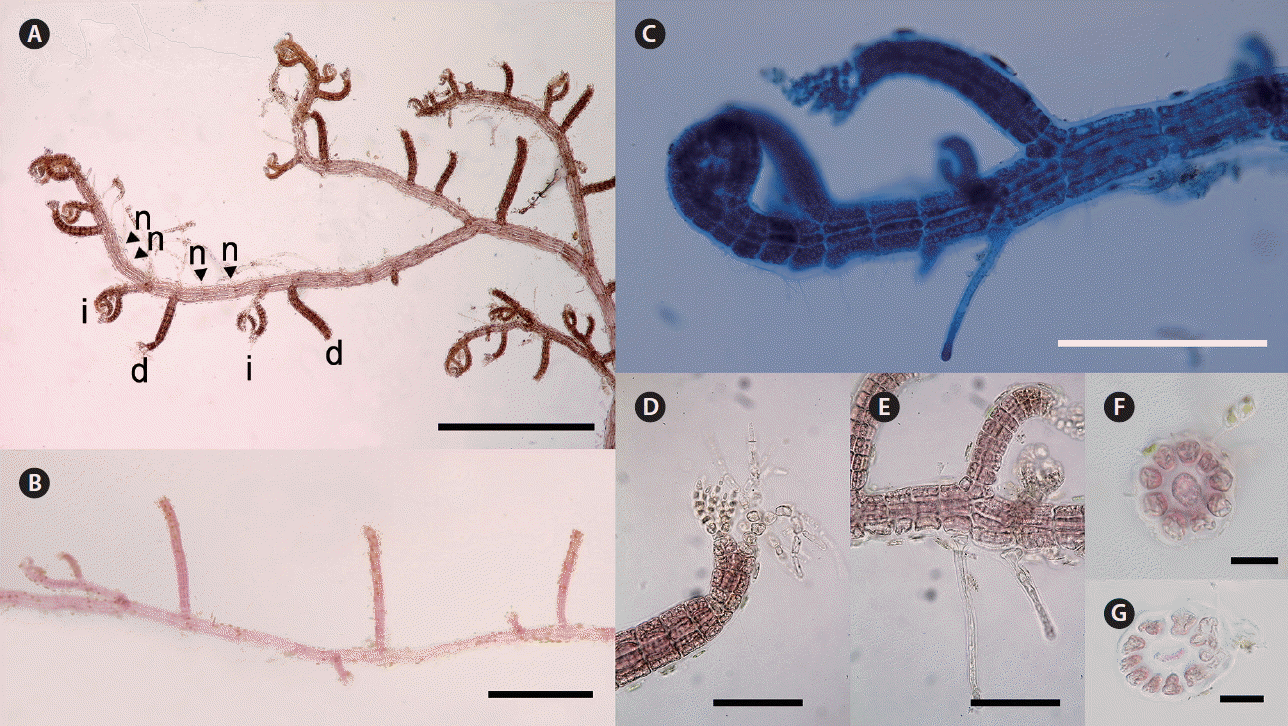
Herposiphonia sparsa sp. nov. Y. H. Koh et M. S. Kim. (A) Thallus collected from Jongdal, Jeju, Korea (d, determinate branch; i, indeterminate branch; n, naked node). (B) Branches showing d/i branching pattern. (C) Apical part of main axis. (D) Apical part of a determinate branch with two vegetative trichoblasts. (E) Rhizoids cut-off from pericentral cells. (F) Cross section of a determinate branch. (G) Cross section of a primary axis. Scale bars represent: A & B, 400 μm; C, 200 μm; D & E, 100 μm; F, 20 μm; G, 40 μm.
Isotype
140704-67, deposited in National Institute of Biological Resources (KB: NIBRAL0000152593).
Type locality
Jongdal, Jeju, Korea (33°29′42″ N, 126°54′45″ E).
Etymology
The specific epithet (sparsa) was chosen to represent the feature of sparse branch development.
Korean name
외쪽거미줄.
Molecular vouchers
MF962755-MF962758 (rbcL); MF962811-MF962813 (COI-5P).
Other specimens examined
130329-6 (Sagye, Jeju, Korea; Mar 29, 2013); 130405-1 (Sagye, Jeju, Korea; Apr 5, 2013); 130724-1 (Jongdal, Jeju, Korea; Jul 24, 2013); 140423-1 (Gimnyeong, Jeju, Korea; Apr 23, 2014); 140603-1 (Mureung, Jeju, Korea; Jun 3, 2014); E14043 (Seongsan, Jeju, Korea; Jun 27, 2014).
Description
Thalli are delicate and red to brown red in color. Primary axes are prostrate, with upwardly circinate apices, attached to other algal species such as Amphiroa spp. and Gelidium spp. by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 5C & E). Prostrate branches are terete, with 10–12 pericentral cells. They are 80–100 μm in diameter and segments are 100–160 μm in length, with L : D ratio 0.5–1 (Fig. 5G). Indeterminate branches (i) arise on primary prostrate axes on alternate sides from every fourth segment, with a determinate branch (d) and two naked nodes (n) on intervening segments in the dorsal position (d/i pattern) (Fig. 5A & B). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary (Fig. 5A–C). Determinate branches are terete, with 8–10 pericentral cells and are 8–10 segments long (Fig. 5F). They are 40–60 μm in diameter with segment L : D ratio 0.2–0.6 (Fig. 5A–C & F). One or two vegetative trichoblasts usually grow at the tip of the determinate branch (Fig. 5D).
Remarks
H. sparsa sp. nov. has been misidentified as H. secunda in Korea due to its d/i branching pattern (Lee 2008). H. secunda is characterized by a d/i branching pattern with 1–4 naked nodes (Ambronn 1880, Hollenberg 1968, Schneider and Searles 1997). In the original description of H. secunda, the number of naked node usually varied between two and three (Ambronn 1880) while H. sparsa has a quite regular d/i/n/n branching pattern of determinate and indeterminate branches. Hollenberg (1968) observed the isotype of H. secunda collected from Tangier, Morocco and described d/i branching pattern with 2 naked nodes. During this study, we collected Herposiphonia specimens from Tarifa, Spain, near of Tangier, which specimens are having same branching pattern and similar morphology with H. secunda. However, we confirmed the morphological differences between H. sparsa and Spanish sample by number of pericentral cell in main axes (10–12 in H. sparsa vs. 8–9 in Spanish one) and number of segment in determinate branches (8–10 in H. sparsa vs. 10–12 in Spanish one). In addition, H. sparsa and Spanish specimens were clearly distinguished the molecular analyses using COI-5P and rbcL gene (data not shown). In the comparisons of other vegetative features, H. sparsa has slenderer (40–60 vs. 60–110 μm) and shorter (8–10 segments vs. 12–25) determinate branches than H. secunda (Hollenberg 1968, Schneider and Searles 1997, Xia 2011, Silva and Fujii 2012). H. donghaensis also has a d/i branching pattern, but it has one or two naked nodes between each d/i set irregularly. In the molecular analysis using COI-5P marker, H. sparsa appeared as a monophyletic group with strong support, clearly separated from H. secunda f. tenella (C. Agardh) Wynne from Spain and Portugal with 15.2–16.6% sequence divergence (Díaz-Tapia and Bárbara 2013). H. sparsa formed a clade with an unidentified Hawaiian Herposiphonia species (HQ422856, HQ423007) with negligible sequence divergence (0.5–0.8%) (Sherwood et al. 2010). Five species, having naked nodes in branching pattern, had been reported in Hawaii, such as H. arcuata, H. dubia, H. obscura Hollenber, H. pacifica, and H. variabilis (Abbott 1999). However, H. sparsa differs from Hawaiian species by several vegetative features such as branching pattern (2 naked nodes regularly in H. sparsa vs. 1 to several naked nodes irregularly in all Hawaiian species), diameter of determinate branches (40–60 μm in H. sparsa vs. over than 60 μm in H. arcuata, H. dubia, H. pacifica, and H. variabilis) and number of segments in determinate branches (8–10 in H. sparsa vs. more than 10 in H. arcuata, H. dubia, H. pacifica, and H. variabilis) (Hollenberg 1968, Abbott 1999). In conclusion, the exact identity of the Hawaiian Herposiphonia species could be confirmed using further morphological and molecular comparisons.
Herposiphonia caespitosa Tseng
Type locality
Putoi Island, Hong Kong.
Korean name
잔디거미줄.
Molecular vouchers
MF962759-MF962760 (rbcL); MF962803-MF962805 (COI-5P).
Description
Thalli are robust and red to brown red in color. Primary axes are prostrate, with upwardly circinate apices, attached to coralline algae by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 6G–I). Prostrate branches are terete, with 10–12 pericentral cells. They are 120–150 μm in diameter and segments are 100–120 μm in length, with L : D ratio 0.5–1 (Fig. 6A). Indeterminate branches (i) arise on primary prostrate axes on alternate sides from every fourth segment, with three determinate branches (d) on intervening segments in the dorsal position (d/d/d/i pattern) (Fig. 6A). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary (Fig. 6A). Determinate branches are terete, with 10–12 pericentral cells and are 20–30 segments long. They are 50–100 μm in diameter with segment L : D ratio 0.5–1.0 (Fig. 6F). One or two vegetative trichoblasts usually grow at the tip of determinate branch (Fig. 6B–D).

Herposiphonia caespitosa Tseng. (A) Vegetative thallus showing d/d/d/i branching pattern (d, determinate branch; i, indeterminate branch). (B & C) Trichoblasts well developed at tips of determinate branches. (D) Apical parts of determinate branches showing scar cells (arrowhead). (E) Determinate branches slightly strict on nodes (arrows). (F) Cross section of a determinate branch. (G) A rhizoid cut-off from pericentral cells. (H) Ends of rhizoids revealing digitate haptera structure. (I) A hapteron attached to Laurencia spp. Scale bars represent: A & B, 400 μm; C, 200 μm; D & E, 100 μm; F–I, 40 μm.
Remarks
First established by Tseng (1943) from Hong Kong, H. caespitosa is characterized by d/d/d/i branching pattern with 3–4 well developed vegetative trichoblasts that are subdichotomously branched 5–6 times, and cystocarps on terminal portions of determinate branches (Tseng 1943). H. caespitosa appears to be closely related to H. elongata M. Masuda & K. Kogame, H. secunda f. densa (Pilger) M. J. Wynne, and H. secunda f. tenella (Tseng 1943, Masuda and Kogame 2000). However, H. elongata is characterized by its spermatangial branches terminated by sterile filaments of five to seven cells (Masuda and Kogame 2000). H. secunda f. densa has distinctly short cystocarpic branches because the apical growth of fertile branches is truncated (Tseng 1943). H. secunda f. tenella has one or two distinctly short vegetative trichoblasts formed spirally on determinate branches that are pseudodichotomously divided one to three times (Wynne 1984, Masuda and Kogame 2000). Lee (2008) previously identified specimens having well developed vegetative trichoblasts at the terminal of determinate branches as H. caespitosa. In this study, we note a close morphological similarity between Chinese and Korean specimens, they have similar d/d/d/i branching pattern, similar number of segments (20–30 in Korean vs. 16–30 in Chinese) and pericentral cells (10–12 in Korean vs. 8–12 in Chinese) in determinate branches, and the number of vegetative trichoblasts (Tseng 1943, Xia 2011). Although we could not confirm molecular monophyly with Korean and Chinese specimens of H. caespitosa, the species is distinctly separated from other species of Herposiphonia in our molecular analyses, with sequence divergences of 13.8% (H. fissidentoides) to 17.4% (H. donghaensis) in COI-5P and 10.9% (H. insidiosa) to 15% (H. subdisticha) in rbcL.
Herposiphonia fissidentoides (Holmes) Okamura
Type locality
Enoshima, Kanagawa Prefecture, Japan.
Korean name
제주거미줄.
Molecular vouchers
MF962761-MF962763 (rbcL); MF962789-MF962791 (COI-5P).
Description
Thalli are robust and red to brown red in color. Primary axes are prostrate, with upwardly circinate apices, attached to other algal species such as Amphiroa spp. or Gelidium spp. by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 7C). Prostrate branches are terete, with 9–12 pericentral cells (Fig. 7D). They are 70–130 μm in diameter and segments are 200–240 μm in length, with segment L : D ratio 1–2. Indeterminate branches (i) arise on primary prostrate axes from alternate sides at every fourth segment with three determinate branches (d) on intervening in the lateral position (d/d/d/i pattern) (Fig. 7A). All determinate and indeterminate branches are laterally arranged (Fig. 7A, B & H). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary (Fig. 7A–C). Determinate branches are ligulate, with 8–16 pericentral cells and are 12–18 segments long (Fig. 7A). They are 80–200 μm in diameter with segment L : D ratio 0.2–0.5 (Fig. 7E–G). Vegetative trichoblasts are absent (Fig. 7A & B). One or two spermatangial branches with a sterile tip are formed on the tip of determinate branches, which are spirally arranged with 50–60 μm in diameter and 140–210 μm in length (Fig. 7I). Tetrasporangia, appearing inside determinate branches, grow to ca. 50 μm in diameter filling up a series of 2–4 segments in lower or middle part of determinate axes during maturation (Fig. 7H).

Herposiphonia fissidentoides (Holmes) Okamura. (A) Vegetative thallus showing d/d/d/i branching pattern (d, determinate branch; i, indeterminate branch). (B) Apical part of main axis. (C) Rhizoids cut-off from pericentral cells. (D) Cross section of primary axis. (E–G) Cross sections of determinate branches. (H) Straight arrangement of tetrasporangia along determinate branches. (I) Terminal portions of spermatangial branches on fertile branches. Scale bars represent: A & H, 200 μm; B, C & I, 100 μm; D–G, 40 μm.
Remarks
In addition to the sequence of determinate and indeterminate branches, their 3-D disposition is also important in the determination of branching patterns for Herposiphonia taxonomy (Masuda et al. 2006). H. fissidentoides displays the d/d/d/i branching sequence, its determinate branches grow on alternate sides of the main axis, forming an almost 180° angle between two consecutive branches (Masuda et al. 2006). This differs from other Herposiphonia species with d/d/d/i branching sequence such as H. prorepens (Harvey) Schmitz where determinate branches are usually arranged in two rows on the same dorsal side of the main axis forming an acute angle between them (Schneider and Searles 1997). Furthermore, while other Herposiphonia species of similar branch arrangement exhibit terete determinate branches (Okamura 1899, Falkenberg 1901, Millar 1990, Norris 2014), our specimens display ligulate shape like Japanese H. fissidentoides. In addition, many other vegetative features such as numbers of segments and pericentral cells in determinate branches and the diameters of primary axis and determinate branches are also within the morphological variation range of H. fissidentoides (Okamura 1899). In the molecular analysis, H. fissidentoides is distinctly separated from other species of Herposiphonia by sequence divergence from 10.8% (H. insidiosa) to 18.3% (H. donghaensis) in COI-5P and from 7.5% (H. jejuinsula) to 12.1% (H. subdisticha) in rbcL.
Herposiphonia insidiosa (Greville ex J. Agardh) Falkenberg
Type locality
ad oras Indiae Orientalis [India].
Korean name
애기거미줄.
Molecular vouchers
MF962764-MF962767 (rbcL); MF962795-MF962802 (COI-5P).
Description
Thalli are robust and red to brown red in color. Primary axes are prostrate, with upwardly circinate apices, attached to coralline algae by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 8F). Prostrate branches are terete, with 9–11 pericentral cells (Fig. 8D). They are 80–100 μm in diameter and segments are 120–180 μm in length, with segment L : D ratio 0.7–1.5. Indeterminate branches (i) arise on primary prostrate axes on alternate sides with some determinate branches (d) on intervening in the dorsal position irregularly but some specimens show a d/d/d/i branch pattern (Fig. 8A). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary. Determinate branches are terete, with 8–9 pericentral cells (Fig. 8E) and are 15–19 segments long (Fig. 8A & B). They are 40–60 μm in diameter with segment L : D ratio 0.5–0.8 (Fig. 8A & E). One or two vegetative trichoblasts grow at the tip of determinate branches (Fig. 8C).
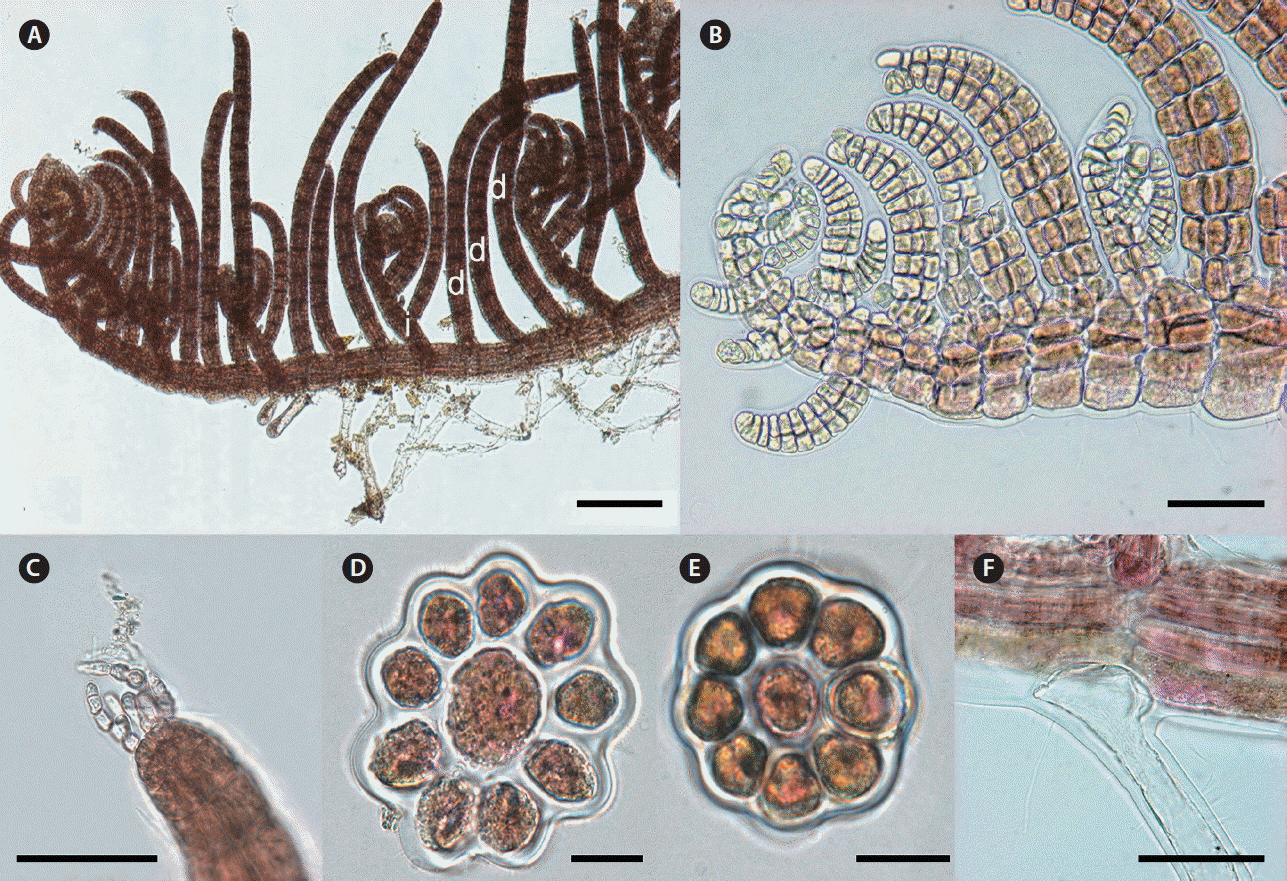
Herposiphonia insidiosa (Greville ex J. Agardh) Falkenberg. (A) Vegetative thallus showing d/d/d/i branching pattern (d, determinate branch; i, indeterminate branch). (B) Apical part of main axis. (C) Apical part of a determinate branch showing a trichoblast. (D) Cross section of primary axis. (E) Cross section of a determinate branch. (F) A rhizoid cut-off from pericentral cells. Scale bars represent: A, 200 μm; B & C, 50 μm; D & E, 20 μm; F, 100 μm.
Remarks
H. insidiosa is known to be widely distributed in the Indo-Pacific oceans including Korea and Japan (Guiry and Guiry 2017). H. insidiosa is characterized by the irregular pattern of indeterminate branches with very closed branches and by forming a thick entangled mass (Okamura 1930, Wynne 1984). Some vegetative features of Korean specimens are well matched with H. insidiosa, such as irregular branching pattern, 8–9 pericentral cells and having one or two vegetative trichoblasts (Agardh 1863, Wynne 1984). However, Korean specimens sometimes show a regular d/d/d/i branching pattern and determinate branches have less segments (15–19 rather than 20–26). In our molecular analyses, H. insidiosa is distinctly separated from other species of Herposiphonia by sequence divergence from 10.8% (H. fissidentoides) to 16.2% (H. sparsa) in COI-5P and from 7.7% (H. jejuinsula) to 11.9% (H. subdisticha) in rbcL.
Herposiphonia parca Setchell
Type locality
Arue Reef, Tahiti.
Korean name
기는거미줄.
Molecular vouchers
MF962773-MF962775 (rbcL); MF962792-MF962794 (COI-5P).
Description
Thalli are delicate and pink red in color. Primary axes are prostrate, with upwardly circinate apices, attached to coralline algae such as Amphiroa spp. by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells (Fig. 9G). Prostrate branches are terete, with 9–10 pericentral cells (Fig. 9E). They are 100–150 μm in diameter and segments are 120–220 μm in length, with segment L : D ratio 1–1.2 (Fig. 9E & F). Indeterminate branches (i) arise on primary prostrate axes on alternate sides from every fourth segment with three determinate branches (d) on intervening in the dorsal position (d/d/d/i pattern) (Fig. 9A & B). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary (Fig. 9A). Determinate branches are terete, with 8–9 pericentral cells and are 12–16 segments long (Fig. 9A & B). They are 55–80 μm in diameter with segment L : D ratio 0.8–1 (Fig. 9A, B & D). Vegetative trichoblasts are absent (Fig. 9C).
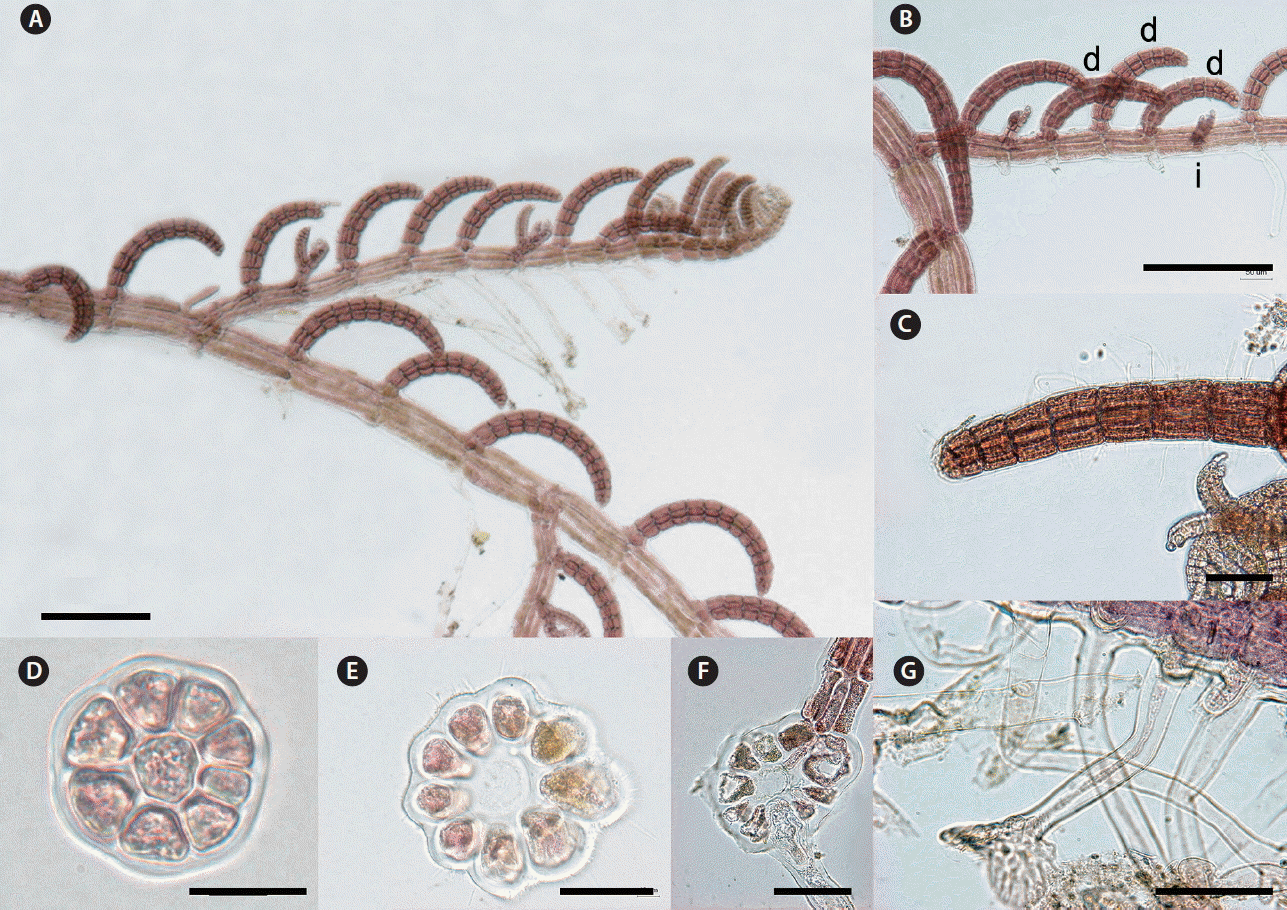
Herposiphonia parca Setchell. (A) Vegetative thallus collected from Sagye, Jeju, Korea. (B) Branches showing d/d/d/i pattern (d, determinate branch; i, indeterminate branch). (C) Apical part of a determinate branch showing absence of vegetative trichoblast. (D) Cross section of a determinate branch. (E & F) Cross section of primary axis. (G) Rhizoids cut-off from pericentral cells. Scale bars represent: A & B, 200 μm; C, F & G, 100 μm; D, 20 μm; E, 40 μm.
Remarks
H. parca is one of the most common species found in the central tropical Pacific Ocean (Hollenberg 1968). This species resembles H. elongata closely in the its branching pattern and thallus dimensions (Masuda and Kogame 2000). H. elongata produces up to four long vegetative trichoblasts per determinate branch in a spiral sequence and they are dichotomously branched. However, H. parca and H. elongata can be distinguished in the location of tetrasporangia, these in H. elongata appearing on distal portions of branches, frequently in spirals (Masuda and Kogame 2000) but, in H. parca, tetrasporangia appear on lower segments of branches (Hollenberg 1968). In morphological comparison between Korean and Hawaiian specimens, Korean specimens have slenderer determinate branches (30–45 μm in Korean) than Hawaiian specimens (50–79 μm) (Hollenberg 1968). In our molecular analyses, Korean H. parca was distinctly separated from other species of Herposiphonia, with sequence divergences of 11.1% (H. fissidentoides) to 18.7% (H. subdisticha) in COI-5P and 8.3% (H. jejuinsula) to 13.2% (H. subdisticha) in rbcL.
Herposiphonia subdisticha Okamura
Type locality
Boshu, Chiba Prefecture, Japan.
Korean name
두줄거미줄.
Molecular vouchers
MF962776-MF962779 (rbcL); MF962806-MF962808 (COI-5P).
Description
Thalli are robust and brown red to brown in color. Primary axes are prostrate, with upwardly circinate apices, attached to coralline algae such as Amphiroa spp. by numerous unicellular rhizoids with digitate haptera cut off from ventral pericentral cells. Prostrate branches are terete, with 10–11 pericentral cells (Fig. 10C). They are 120–150 μm in diameter and segments are 100–180 μm in length, with L : D ratio 0.8–1. Indeterminate branches (i) arise on primary prostrate axes on alternate sides from every fourth segment with three determinate branches (d) on intervening in a lateral position (d/d/d/i pattern) (Fig. 10A). Some indeterminate branches grow like the main axis possessing branches but others remain short or rudimentary (Fig. 10A). Determinate branches are terete, with 8–9 pericentral cells and are 9–12 segments long (Fig. 10A). They are 80–100 μm in diameter with segment L : D ratio 0.8–1 (Fig. 10D). Vegetative trichoblasts are absent (Fig. 10B). One or two spermatangial branches with a sterile tip are formed on the tip of determinate branches, which are spirally arranged with 50–60 μm in diameter and 80–120 μm in length (Fig. 10E).
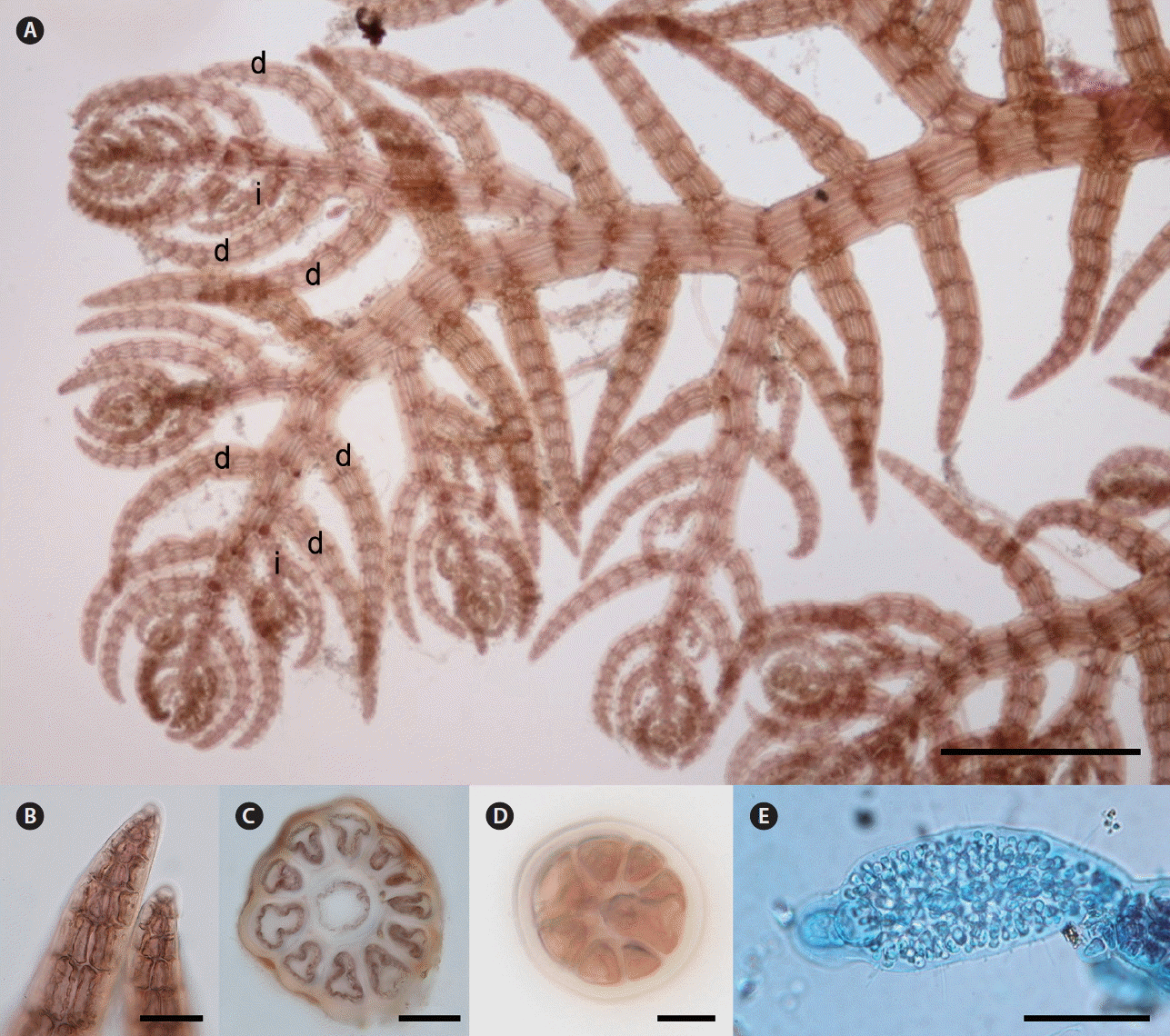
Herposiphonia subdisticha Okamura. (A) Vegetative thallus showing d/d/d/i branching pattern (d, determinate branch; i, indeterminate branch). (B) Apical part of determinate branches without vegetative trichoblast. (C) Cross section of primary axis. (D) Cross section of a determinate branch. (E) Spermatangial branch. Scale bars represent: A, 500 μm; B, C & E, 50 μm; D, 10 μm.
Remarks
H. subdisticha is characterized by a fully decumbent thallus and lateral branching pattern of determinate and indeterminate branches (Okamura 1899). In fact, specimens of H. subdisticha from northwestern Pacific countries such as Korea, Japan, and China share very similar vegetative characteristics in their branching pattern, number of pericentral cells, and the length-diameter ratio of segments (Okamura 1899, Xia 2011). However, when Hollenberg (1968) described lateral branching specimens of H. subdisticha from Hawaii, he noted the difference between Hawaiian and east Asian populations of the species: Hawaiian populations were more strictly distichous, and had determinate branches curved slightly toward the substratum and apices of indeterminate branches slightly inrolled (Hollenberg 1968). In our molecular analyses, H. subdisticha was distinctly separated from other species of Herposiphonia, with sequence divergence of 14.6% (H. insidiosa) to 18.7% (H. parca) in COI-5P and 11.7% (H. insidiosa) to 15% (H. caespitosa) in rbcL.
CONCLUSION
Despite the proven utility of molecular analyses, the genus Herposiphonia has rarely been studied using both COI-5P and rbcL sequences. This study marks the first examination of the genus Herposiphonia in Korea using both morphology and molecular analyses, revealing a hidden biodiversity. We confirmed the presence of eight species including three new species: H. donghaensis sp. nov. is newly discovered from Korean coasts, and H. jejuinsula sp. nov. and H. sparsa sp. nov., which have previously been misidentified as H. nuda and H. secunda, respectively, but they are recognized as new species based on a result of our molecular investigation. Considering the widespread distribution of Herposiphonia species, especially known as the cosmopolitan species like H. insidiosa and H. parca, future studies aimed at understanding its unambiguous taxonomy and species diversity require both molecular and morphological information relating to species from diverse regions of the world.
SUPPLEMENTARY MATERIAL
List of species used for the molecular analyses in this study including collection information, vouchers and GenBank accession number with references (http://e-algae.org).
ACKNOWLEDGEMENTS
We thank all members of the Molecular Phylogeny of Marine Algae Lab. and Dr. John M. Huisman (Murdoch Univ.) for training about morphological observations and field collection of Herposiphonia in Australia. This work was supported by a grant from National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of Korea (NIBR201401204 for collecting samples), and the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (2017R1A2B4009420 for molecular analyses) of the Republic of Korea.
