Two new species of Pseudostaurosiropsis (Bacillariophyta, Fragilariophyceae) from the United States of America, with taxonomic comments on the genus
Article information
Abstract
Two new species of Pseudostaurosiropsis are described based on material collected from rivers in southern United States of America, P. californicus sp. nov. and P. elaboratus sp. nov. Both species have specific characters that set them apart from taxa currently ascribed to the genus. Pseudostaurosiropsis californicus sp. nov. has lanceolate valves with subrostrate, broadly rounded apices, an axial area at the same level as the virgae in internal and external views and both are at the same level as striae in external view, the spine tips are dentate and their growth is produced by filamentous extensions, and it has equal-sized apical pore fields on both valve extremes. On the other hand, P. elaboratus sp. nov. has heavily silicified valves, the virgae are slender than the striae in outer view and wider in inner view, it only has a single, externally occluded apical pore field on one valve extreme, and the spines have a solid core. All species within Pseudostaurosiropsis are contrasted with one another and unique features are described for each based on literature and newly collected image data from type material. The genus is reconsidered and two distinguishing features are recognized: rotae externally occluding the areolae and areolae that are funnel-shaped. These features are contrasted with those in other genera and additional published species that should be included in Pseudostaurosiropsis are discussed.
INTRODUCTION
The pivotal article by Williams and Round (1988) marked the beginning of a profound taxonomic change for fragilarioid diatoms, a morphologically broad group of organisms that were largely included in the genus Fragilaria Lyngbye. The resurrection of Staurosira Ehrenberg and the erection of Fragilariforma D. M. Williams & Round, Pseudostaurosira D. M. Williams & Round, Punctastriata D. M. Williams & Round, and Staurosirella D. M. Williams & Round, meant the split of the small fragilarioids into these genera, but they soon were determined not to be sufficient to accommodate the variability within the group, as revealed by scanning electron microscopy (SEM). Through the study under SEM of populations of small araphids, which valve features could not be clearly resolved under light microscopy (LM), soon additional genera had to be erected. Thus, Nanofrustulum (Lee, Reimer & McEnery) Round, Hallsteinsen & Paasche, Pseudostaurosiropsis E. Morales, Sarcophagodes E. Morales and Stauroforma Flower, Jones & Round, were described to contain some of those newly found morphological variants (Flower et al. 1996, Round et al. 1999, Morales 2001, 2002).
An effort to mark taxonomic boundaries among these genera was published in Morales et al. (2019b). These authors used distinguishing features that are preliminarily hypothesized as synapomorphies, which could systematically explain relationships and establish firmer distinctions among these small araphid genera (see table 1 in Morales et al. 2019b).
After the taxonomic changes triggered by the Williams and Round (1988) revision of Fragilaria and through detailed studies of taxa with purportedly wide distribution (e.g., Morales et al. 2014), it became evident that the revision had also to be done at the species level. For this the re-analysis of type material became important, as well as the study of current populations from different geographic areas. Thus, re-examination of type material for widely reported taxa such as Fragilaria atomus Hustedt (= Stauroforma atomus [Hustedt] Talgatti, C. E. Wetzel, E. Morales & L. C. Torgan) (Talgatti et al. 2014), Fragilaria brevistriata Grunow (= Pseudostaurosira brevistriata [Grunow] D. M. Williams & Round (Morales et al. 2015), Fragilaria nitzschioides var. brasiliensis Grunow (= Fragilariforma brasiliensis [Grunow] P. D. Almeida, C. E. Wetzel, E. Morales & D. C. Bicudo) (Almeida et al. 2017), and Fragilaria elliptica Schumann (= Pseudostaurosira elliptica [Schumann] Edlund, E. Morales & Spaulding) (Edlund et al. 2006), among others, became crucial to resolve species boundaries and triggered the description of several other species (e.g., in the genus Fragilariforma: Almeida et al. 2017; in Pseudostaurosira: García et al. 2021, etc.).
As these changes at the genus and species levels continue to be made and as material from additional geographical areas is studied using traditional microscopy techniques and molecular tools, a higher diversity is being unraveled. Some of the newly found taxa were ascribed to new genera (e.g., Gedaniella Chunlian Li, A. Witkowski & Ashworth [Li et al. 2018] and Popovskayella Kulikovskiy & Lange-Bertalot [Kulikovskiy et al. 2015]), but these have to be reconsidered in the context of all the information generated in recent decades (Morales et al. 2019b). This generates a healthy “taxonomic argumentation” (Silva in Hegewald and Silva 1988), necessary for the construction of a reliable taxonomic and phylogenetic system for araphid diatoms.
Since its description from North America, Pseudostaurosiropsis (Morales 2001) has been reported from Africa (Marquardt et al. 2021), Asia (Luo et al. 2019, Radhakrishnan et al. 2020), Europe (Grudzinska et al. 2014, Rzodkiewicz et al. 2017, Novais et al. 2020) and South America (Seddon et al. 2011, Benito et al. 2015, da Rosa and García 2015, Fayó et al. 2020). To date, this genus contains three species (see later) that share the characteristic of having round areolae externally occluded by rotae, a flat disk-like structure that originates from the base of the spine (Morales 2001, Morales et al. 2019b).
During a nationwide survey of diatoms of the continental United States within the National Water Quality Assessment Program (NAWQA), dependent of the United States Geological Survey (USGS), five populations of Pseudostaurosiropsis were found in rivers of the south. Three of these populations were already discussed in Morales (2001, P. connecticutensis E. Morales, the generitype), Morales (2002, P. geocollegarum [A. Witkowski] E. Morales) and in Morales (2005, P. triradiatum [E. Morales] Kulikovskiy, Glushchenko & B. Karthick, as P. geocollegarum fo. triradiatum E. Morales). Nevertheless, additional SEM images from type material are presented herein for these three taxa. The remaining two populations are proposed here as new species, they are compared with the known species in the genus and comments on the latter and its morphological distinction from other small araphid genera are made.
MATERIALS AND METHODS
Samples were collected as part of the NAWQA Program (Table 1). Water chemistry and biological samples were collected following methodology presented by Fitzpatrick et al. (1998) and Moulton et al. (2002) and are available from https://www.waterqualitydata.us/portal/. Each sample was fixed in the field with formaldehyde (4% final concentration). When chemistry data was not available for the same date of collection of the biological sample, data for the nearest date was chosen from the database (date indicated below, under “Ecology and distribution”).
A subsample of 10–20 mL of each composite sample was digested with nitric acid, rinsed with distilled water to achieve neutrality and, after air drying aliquots on coverslips, they were mounted using Naphrax (Charles et al. 2002). A minimum of 600 valves were counted and identified to the lowest taxonomic level possible at 1,000× under a Nikon Microphot-FXA microscope (Nikon Instruments Inc., Melville, NY, USA) equipped with differential interference contrast and a Spot Insight QE Model No. 4.2 color digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA). Relative abundances of taxa are expressed as a percentage of the total number of individuals counted. Fifty individuals of each population were measured to obtain the size and stria density ranges for each of the taxa ranges.
For SEM studies, aliquots of processed material were air dried onto 15 × 15 cm pieces of aluminum foil. The foil was trimmed into smaller pieces and mounted on aluminum stubs with double-sided tape. The stubs were then coated with gold-palladium using a Polaron Sputter Coater (Quorum Technologies, East Sussex, UK) for ca. 1.5 min at 1.8 kV to achieve a coating thickness of ca. 30 nm. A Leo-Zeiss 982-DSM electron microscope (Carl Zeiss, Halbergmoos, Germany) was used for SEM analysis with an accelerating voltage of 2–4 kV and 10 mm distance.
Samples from Avery Pond corresponded to sediment collected with a Glew gravity corer by Dr. P. A. Siver and collaborators (Connecticut College, New London) following methodology detailed in Marsicano and Siver (1993). Material analyzed for the present work came from the top 1 cm of the core (Table 1). Subsamples (0.5–1.0 g) were digested with an acid-dichromate solution to eliminate organic matter (Battarbee 1986, Marsicano and Siver 1993). Siliceous remains were washed several times with distilled water, small portions were allowed to air dry on coverslips and then mounted for LM and SEM analyses as described above for NAWQA samples.
Archived material and permanent slides are deposited in the Diatom Herbarium, Academy of Natural Sciences of Drexel University, Philadelphia (formerly ANSP). All images were compiled in plates using Adobe Photoshop CS3 v. 10.0 (Adobe Systems, San Jose, CA, USA). Morphological terminology follows Anonymous (1975), Ross et al. (1979) (both references used for terminology applied to striae, areolae and spines), Barber and Haworth (1981) (for terminology related to valve shape and striae orientation), Williams and Round (1988) and Round et al. (1990) (both references used for terminology on areolar substructures, girdle band features and apical pore field characteristics).
RESULTS
Pseudostaurosiropsis californicus E. Morales sp. nov. (Fig. 1A–D [LM], 1H–M [SEM], compare to P. geocollegarum: Fig. 1E–G)
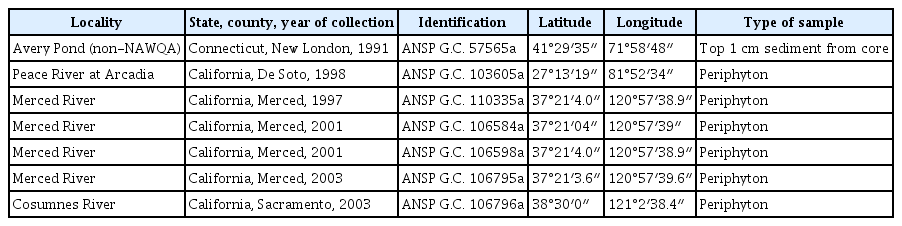
Light microscopy (LM) and scanning electron microscopy (SEM) images of Pseudostaurosiropsis californicus sp. nov. and P. geocollegarum. (A–D) LM valve views arranged in a diminution series of P. californicus sp. nov. from type material G. C. 106598a. (A) Holotype. (E–G) Valve views arranged in a size diminution series of P. geocollegarum from the same material. (H–M) SEM of P. californicus sp. nov. (H) External view of whole valve. Black arrowheads depict hollow spines (G. C. 110335a). (I) Entire frustule in tilted view. Notice spines in formation, with lateral expansions or filaments indicated by striped arrows (G. C. 106598a, type material). (J) Internal view of whole valve. White arrowheads point to internal openings of apical pore fields (G. C. 106796a). (K) Girdle view of tip of a valve with girdle still attached. White arrows show rotae and outer opening of funnel-shaped areolae. Black arrow depicts open end of copula (G. C. 110335a). Striped arrows indicate lateral filaments produced during spine growth. (L) Outer view of tip of a valve. Black arrowheads show solid base of spines. White arrowhead points to external opening of apical pore field. White arrows indicate rotae and opening of funnel-shaped areolae (G. C. 106584a). (M) Side view of two frustules still connected. Black arrows point to open side of valvocopulae (G. C. 106598a, type material). Scale bars represent: A–G, 10 μm; H–K & M, 2 μm; L, 0.5 μm.
Holotype
Academy of Natural Sciences of Drexel University (formerly ANSP) G. C. 106598a (Fig. 1A).
Type locality
United States of America, California, Merced County, Merced River, periphyton depositional (37°21′4.0″ N, 120°57′38.9″ W). Leg. National Water Quality Program, United States Geological Survey, Sep 18, 2001.
Etymology
The epithet “californicus” refers to the State in which the new taxon was found.
Ecology and distribution
The new species was found in a depositional targeted habitat (mix of epipelon and epipsammon). Chemistry data correspond to Sep 10, 2001. Water temperature was 19.5°C, pH 7.9, electrical conductivity 260.0 μS cm−1, and dissolved oxygen 9.0 mg L−1. From the laboratory analyses, orthophosphates were 0.03 mg L−1, and nitrates 0.02 mg L−1. Following Van Dam et al. (1994), the new species could be characterized as alkaliphilous and meso- to oligotraphentic, however, its abundance in the type sample is very low.
Description
Frustules rectangular in girdle view (Fig. 1I & M), forming chains by interlocking spines (Fig. 1M). Valves lanceolate with broadly rounded to subrostrate apices (Fig. 1A–D). Length 9–13 μm, width 3.0–3.5 μm, striae 14–18 in 10 μm. Axial area wide, broadly lanceolate (Fig. 1A–D); externally and internally at the same level of virgae, externally also at the same level of striae (Fig. 1H–J & L). Striae with 2, rarely 3, rows of apically elliptical to round, shallow funnel-shaped areolae (the shape of an inverted isosceles trapezoid in cross section) (Fig. 1I, K & L). All areolae within a stria open into single, shortly elliptical internal depression running from valve face to mantle (Fig. 1J). Rotae roundish to heart-shaped (Fig. 1H, I & K–M). Volae not observed, but siliceous depositions present and filling the depression into which areolae open internally (Fig. 1J). Virgae flared on both extremes; wider than striae (Fig. 1H–L). Vimines slender and short (Fig. 1L). Blister-like depositions present on the abvalvar border of the mantle including the valve apices, elongated and irregular (Fig. 1I & K). Spines originating from vimines at the valve face / mantle junction; softer core at base, somewhat eroded in broken spines, with elliptical base of about the same width as the vimines they sit on (Fig. 1H, I & K–M). Spine body slightly apically flattened and convex on its sides, hollow (Fig. 1H & I). Spine tip variable in shape, generally dentate with an extra lateral, perpendicular extension on each side (Fig. 1M). Growth of spine accomplished by lateral filament-like extensions (Fig. 1I & K). Apical pore fields present on both valve apices (Fig. 1J), composed of 2–3 round poroids (Fig. 1L) and open internally into a circular depression (Fig. 1J). Girdle elements variable in number, open, ligulate and lacking pores (Fig. 1I & K). Valvocopula larger than rest of girdle elements and also open (Fig. 1M).
Accompanying flora
Pseudostaurosiropsis californicus sp. nov. did not appear in a count of 600 valves of the Merced River sample. The diatom community was dominated by Staurosira venter (Ehrenberg) Cleve & Möller (81.5%), Nitzschia palea (Kützing) Smith (3.4%), Planothidium rostratum (Østrup) Lange-Bertalot (1.8%), Placoneis clementis (Grunow) E. J. Cox (1.7%), Navicula viridula var. linearis Hustedt (1.3%), Navicula minima Grunow (sensu lato, 1.2%), and other species with abundances <0.7%.
Pseudostaurosiropsis elaboratus E. Morales sp. nov. (Figs 2A–F [LM], 2L–Q, 3E [SEM], compare to P. connecticutensis: Fig. 2G–K)

Light microscopy (LM) and scanning electron microscopy (SEM) images of Pseudostaurosiropsis elaboratus sp. nov. and P. connecticutensis. (A–F) LM valve views arranged in a diminution series of P. elaboratus sp. nov. from type material G. C. 106796a. (B) Holotype. (F) Short chain with three frustules. (G–K) Valve views arranged in a size diminution series of P. connecticutensis from type material G. C. 57565a. (L–Q) SEM images of P. elaboratus from type material G. C. 106796a. (L) Tilted view of entire frustule. White arrows point to rotae and outer opening of funnel-shaped areolae. Black arrowheads show solid base of spines. Black arrow points to occluded outer opening of apical pore field. (M) Detail of rotae and valve mantle on a tilted valve tip. (N) Internal view of valve. White arrowhead points to internal opening of apical pore field. (O) Dislodged girdle showing open ends of valvocopula (black arrows). Notice lack of fimbriae and smooth advalvar border of pars interior of valvocopula. (P) Tilted view of girdle showing open ends of girdle elements (black arrows). (Q) Short chain with four complete attached frustules. Scale bars represent: A–K, 10 μm; L, N & O, 2 μm; M & P, 1 μm; Q, 5 μm.

Scanning electron microscopy (SEM) images of Pseudostaurosiropsis connecticutensis, P. elaboratus and P. geocollegarum. (A–C) P. connecticutensis from type material G. C. 57565a. (A) Top view of valve. Black arrowheads show hollow spines. White arrowheads point to apical pore fields. (B) Internal view of valve. Reticulated arrows point to common depression into which all areolae within a stria open interiorly. (C) Detail of valve tip. White arrows show rotae and openings of funnel-shaped areolae. Black arrowheads point to solid base of spines. (D) Lateral view of P. connecticutensis. Thick white arrows point to valvocopulae lacking fimbriae and having a smooth advalvar border of the pars interior (G. C. 110335a). (E) Top view of P. elaboratus. White arrows point to rotae and openings of funnel-shaped areolae (G. C. 103605a). (F) Lateral view of P. geocollegarum. White arrows indicate rotae and border of funnel-shaped areolae. Black arrows point to open girdle elements (G. C. 103605a). Scale bars represent: A & D–F, 2 μm; B & C, 1 μm.
Holotype
Here designated, Academy of Natural Sciences of Drexel University (formerly ANSP) G. C. 106796a (Fig. 2B).
Type locality
United States of America, California, Sacramento County, Cosumnes River, periphyton depositional (38°30′0″ N, 121°2′38.4″ W). Leg. National Water Quality Program, United States Geological Survey, Sep 22, 2003.
Etymology
The epithet “elaboratus” refers to the ornate pattern displayed by the spines and valve mantle areolae and their rotae.
Ecology and distribution
The new species was found in a depositional targeted habitat (mix of epipelon and epipsammon). At the time of collection, water temperature was 25.0°C, pH 8.1, electrical conductivity 74.0 μS cm−1, and dissolved oxygen 8.5 mg L−1. From the laboratory analyses, phosphates were 0.06 mg L−1, and total nitrogen 0.02 mg L−1. Following Van Dam et al. (1994), the new species could be characterized as alkalibiontic and oligotraphentic, however, it is not abundant in the sample.
Description
Frustules in girdle view resembling two isosceles trapezoids adjoined by their longer base (Fig. 2F), forming chains by interlocking spines (Fig. 2O & Q). Valves elliptic to broadly elliptic with narrowly to broadly rounded ends (Fig. 2A–E). Length 3–8 μm, width 2–3 μm, striae 14–16 in 10 μm. Axial area wide, broadly lanceolate to elliptic (Fig. 2A–E, L & N); externally and internally faintly depressed with respect to virgae (Fig. 2L–N). Striae with 2–4 rows of apically elliptical to round, shallow funnel-shaped areolae (the shape of an inverted isosceles trapezoid in cross section) (Figs 2L, M, P & 3E). All areolae within a stria open into single, mid-sized internal depression running from valve face to mantle (Fig. 2N). Rotae roundish to heart-shaped (Figs 2L, M, O–Q & 3E). Volae not observed, but siliceous depositions present and filling the depression into which areolae open internally (Fig. 2N). Virgae flared on both extremes of striae due to reduction in the size of the areolae toward the extremes; slender than striae in external view, wider in internal view (Fig. 2L–N). Vimines slender and short (Fig. 2L, M & P). Blister-like depositions present on the abvalvar border of the mantle including the valve apices, short and irregular (Fig. 2M, O & P). Spines originating from vimines at the valve face / mantle junction; solid, with elliptical base of about the same width as the vimines they sit on (Fig. 2L, M, O & Q). Spine body slightly apically flattened, convex on its sides (Fig. 2L, M & O). Spine tip bifurcate with an extra lateral perpendicular extension on each side (Fig. 2L & Q). Apical pore fields present on one extreme (Fig. 2N), occluded externally (Fig. 2L) and open internally into a circular depression (Fig. 2N). Girdle elements variable in number, open, ligulate and lacking pores (Fig. 2L, M & O–Q). Valvocopula larger than rest of girdle elements and also open (Fig. 2M, P & Q), lacking fimbriae (Fig. 2O).
Accompanying flora
Pseudostaurosiropsis elaboratus sp. nov. did not appear in a count of 600 valves of the Cosumnes River sample. The community in the latter was dominated by S. venter (67.3% of relative abundance), N. minima (sensu lato, 5.0%), Cocconeis lineata Ehrenberg (4.5%), Achnanthidium minutissimum (Kützing) Czarnecki (4.3%), Staurosira construens Ehrenberg (4.3%), and other species with abundances <1.2%.
DISCUSSION
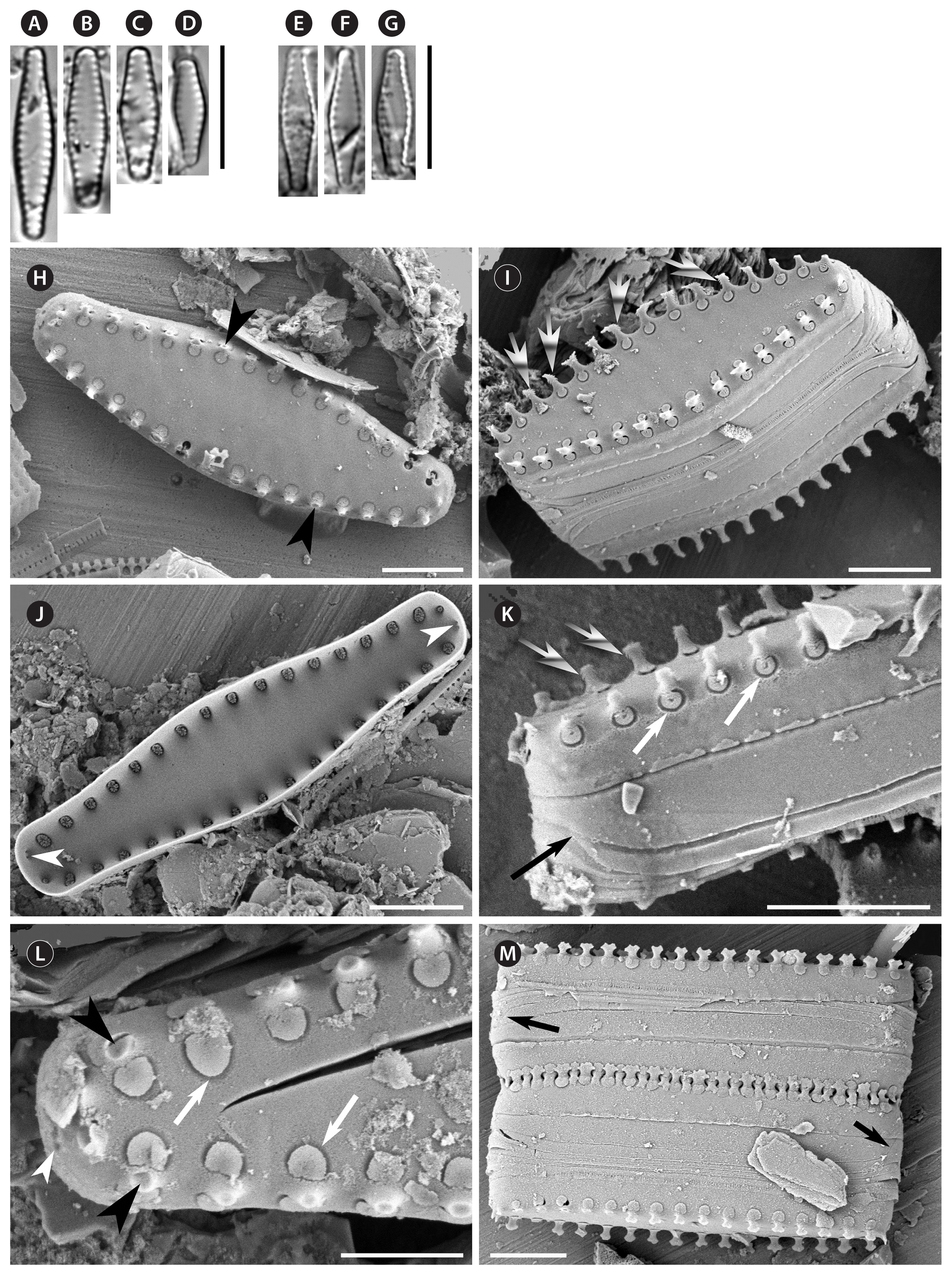
The two new species presented herein fit in Pseudostaurosiropsis, as currently defined, since both have the rotae as unique occlusions in the exterior opening of the areolae (Morales et al. 2019b). Other features such as position and general characteristics of the spines, apical pore fields and girdle bands are also similar to the species currently ascribed to the genus (Table 2).
Comparison among the taxa currently ascribed to Pseudostaurosiropsis, including the two new species described herein
Pseudostaurosiropsis californicus has an overall morphology that resembles P. geocollegarum (compare Fig. 1A–D to Fig. 1E–G). However, as described in Table 2, the latter species has slender valves, which are lanceolate in shape with rostrate ends. Moreover, P. geocollegarum has a configuration of the axial area-striae-virgae complex (see below in Discussion), spine morphology and apical pore field features that are unique and not present on either P. californicus or any of the other three species currently ascribed to the genus Pseudostaurosiropsis (Table 2).
Pseudostaurosiropsis californicus can be distinguished from all other species in its genus by the lanceolate valves with subrostrate ends that end in a broadly rounded apex (compare Fig. 1A–D). The axial area is at the same level as the virgae in internal and external views and both are at the same level as striae in external view (Fig. 1H–L). The spines have tips that are dentate (Fig. 1M) and their growth is produced by filamentous extensions that presumably end up anastomosing and producing the solid structure seen in other valves (Fig. 1I & K). This type of growth has not been seen before, or at least it has not been reported in the published reports of Pseudostaurosiropsis. Similar filamentous extensions, giving the spines a serrate appearance, have also been observed in Nanofrustlum trainori (E. Morales) E. Morales (= Pseudostaurosira trainori E. Morales) (Morales 2001, fig. 6h & i), Pseudostaurosira americana E. Morales (Cejudo-Figueiras et al. 2011, fig. 115), but they are not known to be related to spine growth. Surely, more studies are necessary to determine aspects of spine production and growth in Nanofrustulum, Pseudostaurosira, and Pseudostaurosiropsis.
An additional unique feature in P. californicus is the presence of equal-sized apical pore fields on both valve extremes (Table 2).
The elliptical shape of Pseudostaurosiropsis elaboratus resembles that of P. connecticutensis, however the latter taxon has narrowly rounded valve ends and, considering how refractive they are, the valves of P. elaboratus appear more heavily silicified (compare Fig. 2A–F to Fig. 2G–K). The depth of the external areolar opening in Fig. 2P shows in part the heavy silicification of this taxon.
Apart from the heavy silicification, the distinguishing features of P. elaboratus that set this taxon apart from others in Pseudostaurosiropsis are the virgae that are slender than the striae in outer view, but wider in inner view (Fig. 2L–N), the presence of a single apical pore field on one valve extreme, the solid core of the spines, and the apical pore field that is externally occluded (Table 2).
Comments on key characteristics of Pseudostaurosiropsis
A feature appearing in all species of Pseudostaurosiropsis that has not been discussed in the literature is the funnel-shaped areolae (see them in P. californicus: Fig. 1K & L, white arrows; P. elaboratus: Figs 2L & 3E, white arrows, and Fig. 2M & P; P. connecticutensis: Fig. 3C & D, white arrows; P. geocollegarum: Fig. 3F, white arrows, and P. triradiatum: Fig. 4B & D, white arrows). In each case, areolae start as a shallow depression carved on the valve face surface and which gradually decreases in circumference until the areolar opening border is reached (e.g., Fig. 3C). The areola opens directly to the valve interior with no depression surrounding it (e.g., Fig. 3B). Interiorly, all areolae within a stria open into a single transapical trough or depression, running from valve face to mantle (e.g., Fig. 3B, see also Radhakrishnan et al. 2020, fig. 4a–d) and varying in width, length and overall shape depending on the species (Table 2).
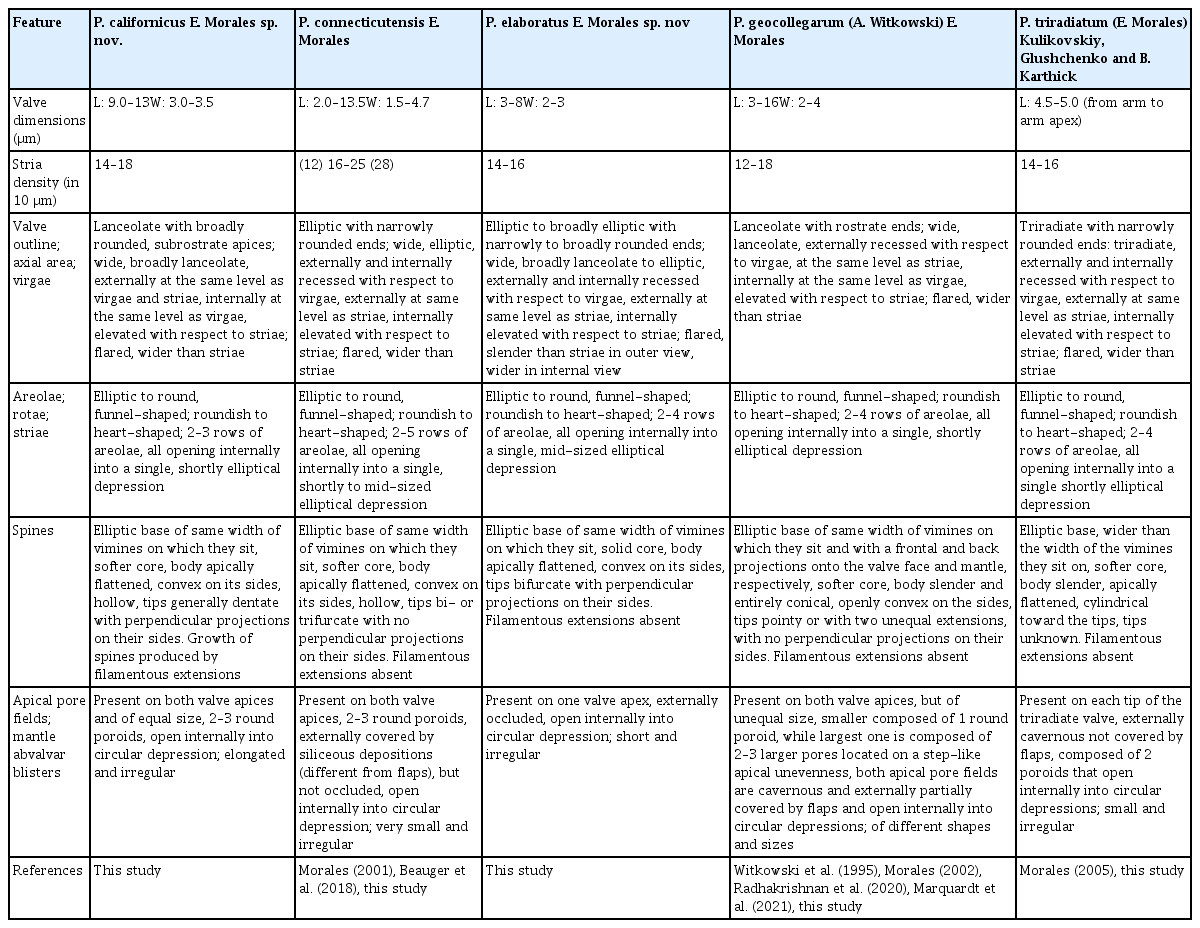
Scanning electron microscopy (SEM) images of Pseudostaurosiropsis triradiatum from type material G. C. 103605a. (A & B) Top view of valves. White arrowhead shows outer opening of apical pore field. White arrows show rotae and opening of the trapezoid areolae. (C) Internal view of valve. (D) Detail of valve surface. Black arrowheads point to hollow spine basis. White arrows show eroded rotae and openings of the trapezoid areolae. Scale bars represent: A–C, 2 μm; D, 1 μm.
The funnel-shaped areolae in Pseudostaurosiropsis have also been observed in some species of Pseudostaurosira, such as P. brevistriata (Morales et al. 2015, figs 128–130), in which especially the first row of mantle areolae show the funnel configuration, but it is often obscured by the spine ligulae and flaps covering the areolae. A similar occurrence of mantle funnel-shaped areolae is that in P. diablarum Seddon & A. Witkowski (Seddon et al. 2016, figs 21 & 23), and in P. zolitschkae M. L. García, S. Bustos, Maidana & E. Morales (García et al. 2021, figs 108 & 110).
A species of Pseudostaurosira that has funnel-shaped areolae on both valve face and mantle is P. elliptica (Edlund et al. 2006, figs 20, 22 & 24), however, each areola has an elevated ring and from there, there is a steeper (than in species of Pseudostaurosiropsis) transition to the border of the areolar opening. In an internal view of all the species of Pseudostaurosira cited above, the areolae do not possess an individual surrounding depression or ring and all the areolae along a stria open into a transapical trough that is continuous from valve face to mantle (see cited references above).
Therefore, although the funnel-shaped occurs in both Pseudostaurosira and Pseudostaurosiropsis, all the species in the latter genus have them, they have an outer shallow depression, and all of the areolae within a stria possess the funnel configuration.
A set of features that is worth exploring is that of the axial area-virgae-striae. As seen in Table 2 the interplay of these three features produces character combinations that can be used to recognize and discriminate at least three of the species within Pseudostaurosiropsis, as shown above for the two new species. In recognizing these character combinations, the elevation of the axial area with respect to virgae and striae, and the elevation and width of virgae and striae have to be observed in both internal and external view. For example, externally in P. geocollegarum the axial area is recessed with respect to virgae and at the same level as striae, while internally the axial area is at the same level as the virgae, and both are higher than the striae (Table 2) (Radhakrishnan et al. 2020, figs 3b & 4a). This combination is unique among the five taxa included in Table 2 and together with the valve outline, spine, and apical pore field morphology and location, they are the key features for the reliable identification of this taxon.
Spines, as seen in Table 2, are another feature for which there is variability in the taxa currently ascribed to the genus, including the two new species described here. While the base of spines tends to be elliptic in all taxa since it is slightly flattened in an apical sense, the width of these base, in the apical sense and with respect to the length of the vimines on which they develop, can extend onto the flanking virgae (as it happens in P. triradiatum) (Fig. 4A, B & D) or they can have the same width as the vimines (in the rest of the species in Table 2). Another variation appears in P. geocollegarum, in which the base has a frontal and back projections, making the spines look sagittate on side view (Radhakrishnan et al. 2020, fig. 3a & b).
The body of the spines also shows variability among the taxa currently included in Pseudostaurosiropsis. While the spine body tends to be, in general, apically flattened, in P. geocollegarum the body is entirely conical instead. In P. triradiatum appears another variation in which the spine body starts as an apically flattened structure (Morales 2005, fig. 132) but then becomes cylindrical toward the top (Fig. 4B & D). The only taxon in which the spine body is solid, denoted by the observable even texture of its core and lack of hollow eroded spines in all specimens observed, is P. elaboratus (Figs 2L & 3E). The remainder of the taxa have a more porous core and in eroded spines a hollow center can be seen. This is the case in P. californicus (Figs 1H; L shows a spine base with softer core, somewhat eroded), P. connecticutensis (Fig. 3A; C shows a softer, somewhat eroded core at spine base), P. geocollegarum (Morales 2002, pl. 2, fig. 2), and P. triradiatum (Fig. 4D).
Finally, the tips of the spines are also variable among the species in Pseudostaurosiropsis. The spine tips in P. triradiatum were not observed and this is probably because they are very thin in such small spines and could be easily lost during preparation. In the rest of the taxa the spine tips are dentate in P. californicus (Fig. 1M), bi- or trifurcate in P. connecticutensis (Morales 2001, fig. 7g & h), exclusively bifurcate in P. elaboratus (Fig. 2L, O & Q) and pointy with two extensions of different size in P. geocollegarum (Radhakrishnan et al. 2020, fig. 2d). It is worth mentioning that the descriptions of spines in Table 2 correspond to what appear to be fully grown spines, usually observed from attached neighboring valves, as is the case of all figures cited above.
Spine structure has also been shown to be variable in other small araphid genera (see discussion in Morales et al. 2019b). The latter authors also discussed the high variability of spines in the genus Pseudostaurosira, which range from absence of these structures to production of hollow or solid spine bodies on either vimines or virgae.
Also, in Staurosirella spine differences have been used to define and differentiate species (Guerrero et al. 2019, table 1, Morales et al. 2019a). In this case, spines can be absent, represented by agglomeration of papillae, or be produced on the virgae, where they can originate from one or two points. In the different species currently ascribed to the genus, spine body and tips assume a plethora of variations that can be used to recognize species, though these features have not been sufficiently explored (Morales and Manoylov 2006).
Spines have also been recognized as variable structures in Fragilariforma (Almeida et al. 2017). These authors recognized a species in which spine production is facultative (e.g., F. constricta (Ehrenberg) D. M. Williams & Round), species with minute or incipient spines (e.g., F. strangulata (Zanon) D. M. Williams & Round), or hollow or solid, well-developed spines with differing morphologies (as in F. telum (J. R. Carter & P. Denny) P. D. Almeida, C. E. Wetzel & E. Morales and Fragilaria brasiliensis (Grunow) P. D. Almeida, C. E. Wetzel, E. Morales & D. C. Bicudo, respectively).
Recognizing all possible variations of spine structure in all small araphid genera is beyond the scope of this manuscript. But, the examples cited thus far, including what was described for species in Pseudostaurosiropsis above, show that spine structure is species specific and can be reliably used to distinguish taxa at this taxonomic level.
Apical pore fields are another structure that exhibit variability among the taxa included in Table 2. As can be seen in this table, at least four of the five species have a unique feature of the apical pore field that distinguishes each taxon. The features considered to describe apical pore fields herein were number of poroids, whether they open to the valve surface (at the mantle) directly or in troughs, whether they were occluded or had flaps, and how the poroids open at the valve interior.
The variability seen in the apical pore fields of Pseudostaurosiropsis has also been observed in Pseudostaurosira in which poroids can open exteriorly in troughs (Morales et al. 2012, fig. 42, Marquardt et al. 2021, figs 48–51), in depressions (Grana et al. 2018, fig. 20), semi-covered by irregular siliceous growths (Cejudo-Figueiras et al. 2011, figs 103 & 104), or covered by flaps (Morales et al. 2015, figs 130 & 138), or be entirely occluded (Morales et al. 2012, fig. 50). Interiorly, the poroids can also open in several ways (see discussion in Morales et al. 2019b and references cited above).
In other small araphid genera such as Staurosirella the variability in the characteristics of the apical pore fields is much reduced (see discussion in Morales and Manoylov 2006) and apical pore fields of the remaining genera mentioned in this manuscript have not been studied in detail, preventing comparative considerations. Therefore, it seems that at least for Pseudostaurosira and Pseudostaurosiropsis apical pore fields are structures that can aid in the distinction of species.
Table 2 includes a comparison of blister development in the abvalvar edge of the valve mantle. However, this is just an initial observation of such structures and only partial evidence of its variability among all taxa was observed. For example, while these structures in a species could be either elongated (as in P. californicus, Fig. 1I, K & M), short (as in P. elaboratus, Fig. 2M & O), or very small compared with these long / short blisters (as in P. connecticutensis, Fig. 3D), they are often irregular in their development and are highly variable regarding their individual shape and size even within the same valve (compare for example valves along the same chain in P. elaboratus, Fig. 2Q). This is why no conclusion has been drawn here regarding this structure.
Another feature that has not been considered in Pseudostaurosiropsis is girdle band structure. Observations on the two new species presented herein and in P. connecticutensis show that the pars interior advalvar border is always smooth (see Fig. 2O for P. elaboratus and Fig. 3D for P. connecticutensis), this is perhaps because the pars interior does not contact the striae and virgae at the valve interior and it attaches directly to the inner wall of the valve mantle. In contrast species of Pseudostaurosira tend to have a wavy advalvar border of the pars interior (see for P. parasitica (W. Smith) E. Morales (Morales et al. 2015, figs. 101 & 102) and for P. sajamaensis E. Morales & Ector (Morales et al. 2012, fig. 49), apparently because they contact the internal elevations of the virgae, though this still needs to be clearly shown, but see for P. decipiens E. Morales, G. Chávez & Ector (Morales et al. 2012, fig. 44, showing a valvocopula still attached).
Despite the above comparison, the literature on girdle bands of both Pseudostaurosira and Pseudostaurosiropsis is scant (this is the reason this structure is not considered in Table 2), preventing any hard conclusions at this point.
Girdle bands in other small araphid genera are insufficiently studied to make fair comparisons (i.e., Fragilariforma, Sarcophagodes and Staurofoma). While in others, girdle band structure is different (i.e., Nanofrustulum has quasifract girdle elements (Grana et al. 2015, figs 16 & 17), Punctastriata (Wetzel and Ector 2021, fig. 77), Staurosira (Morales 2006, figs 22 & 23) and Staurosirella (Morales 2006, figs 32 & 35) all have fimbriate valvocopulae, which are radically different from that in Pseudostaurosiropsis.
In summary, considering the possession of the rotae makes all the species in Table 2 a cohesive group and the funnel-shaped areolae reinforces that grouping at the genus level. While girdle band structure needs to be studied in more detail to be considered as a distinguishing feature at the same level. All of the rest of the features discussed here and included in Table 2 serve well in the distinction of taxa at the species level.
The literature presents additional representatives that present both distinguishing features of Pseudostaurosiropsis and that have been erroneously identified at the species level. Such is the case of the population illustrated by Fayó et al. (2020) identified as P. geocollegarum. This population has a characteristic lanceolate valve shape, but the valves are inflated in the middle, showing conspicuously convex valve sides at the central area. Also, this species has spines that differ from the other taxa included in Table 2, having a body that is notoriously convex on the sides and the ends that are spatulate.
Marquardt et al. (2021) presented an SEM image of a specimen from Lac D’Yrieux, France (fig. 64), which was identified as P. geocollegarum, but that has a width higher than 4 μm, and the rotae are wider than those of P. geocollegarum (ca. 1.7 μm vs. 0.2 μm). Interestingly, this taxon also has an elevated areolar ring (as that discussed for P. elliptica), though very thin, and from there is a gradual decrease in elevation to end at the areolar opening. This feature is clearly shown in the lower right side of the specimen where there is a broken rota (Marquardt et al. 2021, fig. 64). This specimen from France also has spines with an apically flattened body that is notoriously convex on the sides, and apparently the tips are bifurcate.
ACKNOWLEDGEMENTS
I thank Dr. M. Cantino and the late J. Romanow for help during SEM sessions at the Electron Microscopy Laboratory, Department of Physiology and Neurobiology, University of Connecticut. I also thank USGS-NAWQA biologists and personnel for sample and water chemistry collection, and Dr. D. Charles for his support during my stay at ANSP in the period 2000–2006. Special thanks go to Dr. M. Potapova, Curator of the Diatom Herbarium, Drexel University for kindly providing sample information. The author was partially funded by the Portuguese Foundation for Science and Technology (FCT) project UIDB/04683/2020 - ICT (Institute of Earth Sciences), and the Agência Portuguesa do Ambiente, APA-000004DFIN.AALP/2017 integrated within the Operational Program for Sustainability and Efficiency in the Use of Resources 2014-20, POSEUR-03-2013-FC-000001.
Notes
The author declares that he has no potential conflicts of interest.