Effects of light-emitting diodes on protoplast regeneration from gametophytic cells of the commercial kelp Undaria pinnatifida (Laminariales, Phaeophyceae)
Article information
Abstract
Light-emitting-diodes (LEDs) are a lighting source useful for the precise evaluation of light quality effect on biological systems. Despite the importance of light spectra on the regeneration of land plant protoplasts (“naked cells”), this factor has not been tested yet on protoplasts from multicellular algae. This study reports on the effects of pure primary colors (red, blue, and green), dichromatic (red plus blue, RB, 1 : 2) and white LEDs on protoplast regeneration from male and female Undaria pinnatifida gametophytes. We also evaluated the effect of different light spectra on pigment composition (chlorophyll a, chlorophyll c, and fucoxanthine), and the light intensities under the best condition on the regeneration process. In the early stages, blue or RB LEDs increased the percentage of dividing female protoplasts, whereas red, blue, and RB LEDs enhanced that of dividing male protoplasts. In the later stages, RB LEDs showed a positive effect only on the percentage of multiple rhizoid-like protrusions (male gametophyte). They also increased the final area of both regenerated gametophytes. The LEDs did not affect pigment composition in female gametophytes. In male gametophytes, in contrast, they reduced chlorophyll c, while blue, RB, and green LEDs decreased fucoxanthin. Under RB LEDs, the optimal light intensity was 80 μmol photons m−2 s−1 for female gametophytes and 40 to 60 μmol photons m−2 s−1 for male gametophytes. Our results suggest that dichromatic LED illumination (red–blue) improves regeneration of U. pinnatifida gametophyte-isolated protoplasts. Thus, dichromatic LEDs might a suitable light source for enhancing protoplast regeneration in brown seaweeds.
INTRODUCTION
Protoplast regeneration is a process in which a protoplast (“naked” plant cell) resynthesizes its cell wall and undergoes cell division, elongation (growth), and differentiation, resulting in a new plant (Warren 1992, Wang and Ruan 2013, Goldman 2014). This ability is the ultimate test of protoplast viability and is essential for establishing protoplast systems (Bhojwani and Razdan 1996, Chawla 2009). Several factors affect protoplast regeneration ability, such as osmoticum, plating density, culture conditions, and light (Bhojwani and Razdan 1996). Light spectral quality plays an important role in regeneration, and photosynthetic organisms respond to different wavelengths. For example, exposure to white light is often the best way to induce shoot formation in protoplasts of land plants (Compton et al. 2000). Blue and red light increase the number of asymmetric cells in protoplasts of the moss Physcomitrella (Jenkins and Cove 1983), while red light promotes protoplast germination in the unicellular marine green alga Boergesenia forbesii (Ishizawa et al. 1979).
In seaweeds, protoplast regeneration has been accomplished in 40 species (Matsumura et al. 2001, Reddy et al. 2008, Yeong et al. 2008, Lafontaine et al. 2011, Wang et al. 2014, Huddy et al. 2015, Avila-Peltroche et al. 2019, Avila-Peltroche and Won 2020). Although different factors affecting protoplast regeneration in seaweeds have been studied (Reddy et al. 2008), the effect of light spectral quality has never been analyzed.
In marine environments, different light qualities occur within the water column by absorption and scattering of light, depending on the depth, turbidity, time of the day, and other physical parameters. For example, blue-green waveband usually penetrates deepest into the water body due to its shorter and longer wavelength (Hanelt and Figueroa 2012, Falcón et al. 2020). These differences in light colors play an important role in marine macroalgal physiology, especially in kelp gametophytes, with several studies showing that spectral quality can control sexual reproduction and vegetative growth (Edwards 2022).
Light-emitting diodes (LEDs) are a rapidly developing technology consisting of electronic diodes that produce light when an electric current passes through them. They come in the three primary colors and as dichromatic, trichromatic, or white LEDs. LEDs have numerous advantages over conventional lights; for example, the light emission at specific wavelengths allows to precisely evaluate the effect of light quality on biological systems, which is especially important in photosynthesis research (Gupta and Jatothu 2013, Dayani et al. 2016). In seaweeds, the use of LEDs is still limited to a few species and have been focused on growth studies (Murase et al. 2014, Kim et al. 2015, Miki et al. 2017, Le et al. 2018).
In this study, we analyzed the regenerative effect of pure primary color (red, blue, and green), dichromatic (red–blue; hereafter RB), and white LEDs on Undaria pinnatifida protoplasts during early (cell division) and late (cell elongation/growth) development. We isolated the protoplasts from male and female gametophytes because of their high potential to produce bioactive compounds (Dwiranti et al. 2012, Seppic 2022) and ability to be cultured in bioreactors (Rorrer and Cheney 2004, Gao et al. 2005). These features make them suitable for cellular biotechnology techniques, many of which rely on protoplasts (Reddy et al. 2008). We also investigated the effect of light spectral quality on photosynthetic pigments and light intensities under the most favorable wavelengths for regeneration.
MATERIALS AND METHODS
Establishment of gametophyte clones
Undaria pinnatifida gametophyte clones were established from mature sporophytes sampled at Geoje and Jindo Islands, Korea on Mar 1, 2013 and May 5, 2016, respectively. Sporulation, isolation, and culture were performed as previously described (Avila-Peltroche et al. 2020). Female (from Jindo Island) and male (from Geoje Island) vegetative gametophyte cultures in the early (4-day-old) and mid-exponential phase (6-day-old), respectively, were used for subsequent experiments.
Protoplast isolation
Protoplast isolation was performed following the protocols described by Benet et al. (1997) and Coelho et al. (2012) with some modifications (Avila-Peltroche et al. 2020). Approximately 100 to 200 mg of U. pinnatifida gametophytes from 1-L round flasks were incubated in a 0.22 μm filter-sterilized enzymatic solution (640 mM NaCl, 208 mM MgCl2·6H2O, 35.2 mM MgSO4, 256 mM KCl, 3.2 mM CaCl2, 16 mM MES [pH 6], 2,512 mOsm L−1 H2O) at 20°C with shaking at 70 rpm for 4 h (female) or 6 h (male) in the dark. The solution was supplemented with 1% cellulase RS (Yakult Co. Ltd., Tokyo, Japan), 4 U mL−1 alginate lyase, and 1% driselase from Basidiomycetes sp. (Sigma-Aldrich, St. Louis, MO, USA). Chelation pre-treatment was conducted with a calcium-chelating solution (665 mM NaCl, 30 mM MgCl2·6H2O, 30 mM MgSO4, 20 mM KCl, and 20 mM ethylene glycol-bis (β-amino-ethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt (EGTA-Na4) [pH 5.5] as calcium chelator) for 20 min prior to enzymatic digestion (Coelho et al. 2012). Protoplasts were filtered using a 25-μm nylon mesh to remove undigested filaments and further washed three times by centrifugation at 100 ×g for 10 min. The pellets were resuspended in an enzymatic solution. The average number of gametophyte-isolated protoplasts was calculated with a hemocytometer. For male gametophytes, the fresh weight was approximately (3.12 ± 0.51) × 106 protoplasts g−1 and for male gametophytes, (2.11 ± 0.08) × 106 protoplasts g−1. The viability of protoplasts and cell wall regeneration were assessed by red chlorophyll autofluorescence and staining with calcofluor white M2R (Sigma-Aldrich), respectively, as previously described (Avila-Peltroche et al. 2019). Calcofluor white was chosen for monitoring cell wall formation because it binds to β-linked fibrillar polymers such as cellulose but does not block its synthesis (Murgui et al. 1985).
LEDs and growth conditions
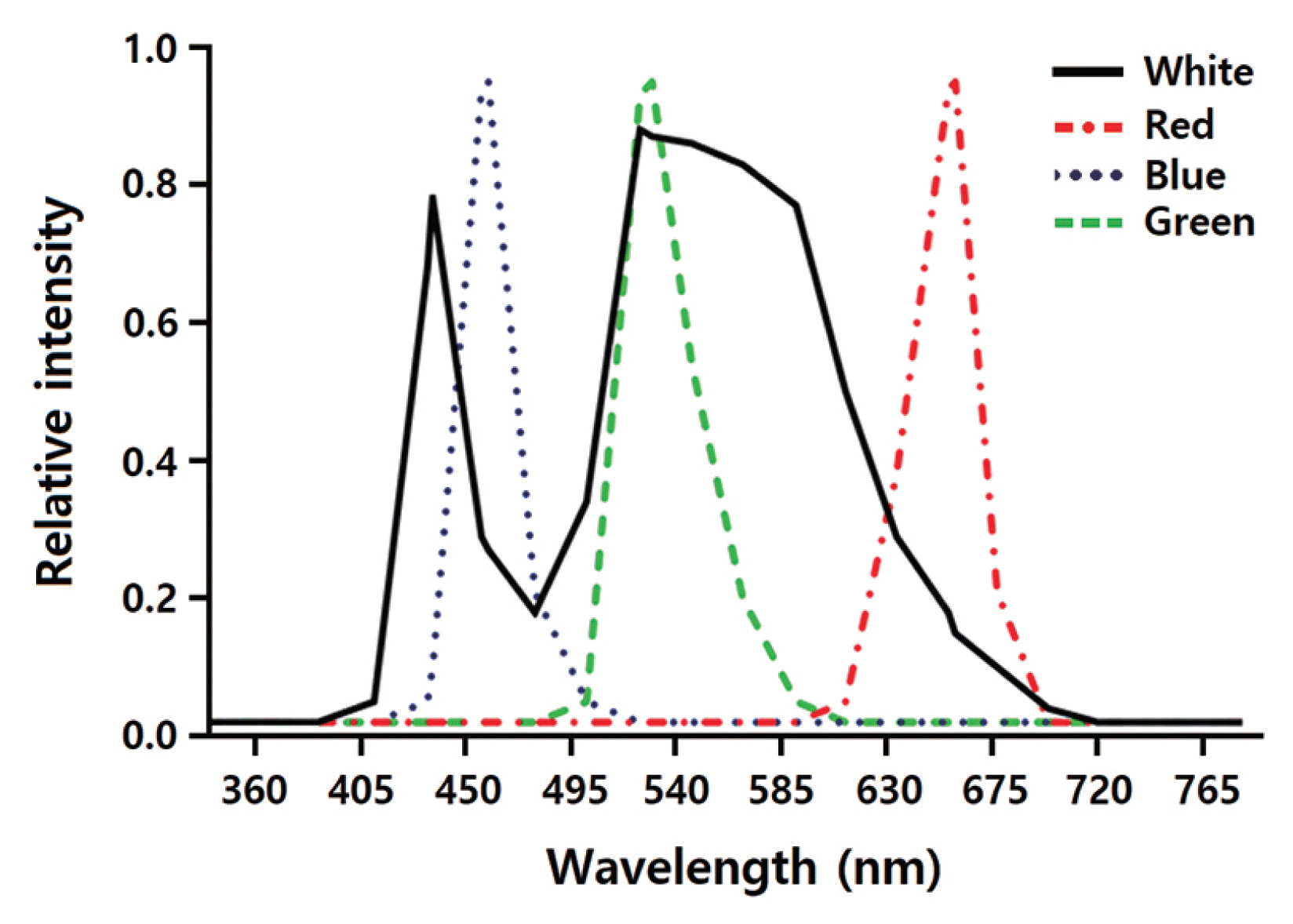
The effects of LED wavelengths on regeneration of the gametophyte-isolated protoplasts were investigated using a DYLED 44V system (DyneBio Co., Seongnam, Korea). Five different LEDs were installed in the growth chambers: (1) white (all wavelengths), (2) red (peak wavelength at 660 nm), (3) blue (peak wavelength at 460 nm), (4) mixed red and blue (1 : 2; RB), and (5) green (peak wavelength at 530 nm). Fig. 1 shows the emission spectra of the LEDs. Spectral wavelengths were measured using the USB2000 UV-VIS spectrometer (Ocean Optics Inc., Dunedin, FL, USA), while the LED irradiation intensities were measured using a quantum meter (MQ-500; Apogee Instruments, Logan, UT, USA). The protoplasts were grown under LEDs on a 14 : 10 h light : dark cycle.
Protoplast regeneration under different LEDs
Protoplasts were dispensed into at least 4 wells of a 12-well tissue culture plate containing 2 mL of regeneration medium (PES with 285 mM NaCl and 5 mM CaCl2). The medium was supplemented with an antibiotic mix (50 mg L−1 penicillin G, 25 mg L−1 streptomycin, and 5 mg L−1 chloramphenicol). The plates were incubated at 20°C in the dark and with an initial protoplast density of 9 × 103 protoplasts. Previous experiments showed that 20°C was suitable for successful protoplast regeneration. Also, this temperature promotes vegetative growth of U. pinnatifida gametophytes (Morita et al. 2003). After 4 days in the dark, osmotic pressure was reduced slowly using a PES medium, and cultures were gradually exposed to a final intensity of 40 μmol photons m−2 s−1. The well plates were separately irradiated using white (control), red, blue, RB, or green LEDs. The culture medium was replaced weekly. The responses of cultured protoplasts were assessed during the early and late stages of regeneration. In the early stages, the percentage of dividing cells was calculated at 8 (female) and 11 (male) days in culture. In addition, the percentage of asymmetric cells formed by cell divisions was determined for all conditions because LED lighting promotes the formation of asymmetric cells in protoplasts of the moss Physcomitrella (Jenkins and Cove 1983). The percentage of branched filaments (BFs) or multiple rhizoid-like protrusions (MRLPs) was determined at 15 or 18 days in culture, respectively. Final growth was represented as the gametophyte area at the end of the experiment (female = 22 days in culture; male = 26 days in culture) and analyzed using image analysis software (ImageJ; US National Institutes of Health, Bethesda, MD, USA). For percentage calculations, at least 100 cells per well were counted. For area measurements, at least 30 gametophytes per well were analyzed.
Pigment extraction and quantification
Chlorophyll a (Chl a), chlorophyll c (Chl c), and fucoxanthin (Fx) were extracted from regenerated female gametophytes (10–20 mg fresh weight) at the end of the experiments using the protocol and equations of Seely et al. (1972) with dimethyl sulfoxide as the solvent. Regenerated male gametophytes were further cultured under the same conditions for 3 months to obtain sufficient biomass for reliable measurements. A microplate reader (SpectraMax Paradigm; Molecular Devices, San Jose, CA, USA) was used to quantify the pigments from at least three wells per LED treatment. Pathlength correction was performed according to the method described by Warren (2008).
Protoplast regeneration under different LED intensities
The effect of LED light intensity on protoplast regeneration was evaluated under RB LED treatment. The gametophyte-isolated protoplasts were cultured as described above in at least 4 wells of a 12-well plate. They were placed at different distances from the LED unit to achieve different intensities: 20, 40, 60, and 80 μmol photons m−2 s−1. The light intensities were measured using a quantum meter. The regenerative responses of the cultured protoplasts were assessed as previously described.
Statistical analysis
The percentage of dividing cells, asymmetric cells, BFs, or MRLPs, were analyzed as proportions with beta regressions since beta distribution provides a flexible model for continuous variables restricted to the interval (0, 1) (Ferrari and Cribari-Neto 2004). The analyses were performed using the R package betareg (Cribari-Neto and Zeileis 2010). The proportional reduction of error (PRE) statistic was used as the overall model effect size (Smithson and Verkuilen 2006).
Two separated one-way ANOVA tests were used to evaluate the differences in gametophyte area and pigment composition. Normality and homoscedasticity were checked using the Shapiro-Wilk and Levene test, respectively, before conducting ANOVA. Kruskal-Wallis or Welch’s ANOVA tests were used when data did not meet normality or homoscedasticity assumptions, respectively. Effect sizes (Sullivan and Feinn 2012) were presented as ω2 (Field 2009, Lakens 2013) in the case of significant results. The formula for ω2 calculation was as follows:
, where SSM is the between-group effect, dfM is the degrees of freedom for the effect, MSR is the regression mean square, and SST is the total amount of variance in the data. All the analyses were performed using the car (Fox and Weisberg 2019) and userfriendlyscience packages (Peters 2018) in R.
Tukey’s post hoc test was used when the results were significant. When data were heteroscedastic, the Games-Howell test was performed instead. The post hoc comparisons were conducted using the multcomp (Hothorn et al. 2008) or userfriendlyscience package (Peters 2018) in R.
The significance threshold was set at p = 0.01 to reduce the true type I error rate (at least 7% but typically close to 15%) (Sellke et al. 2001). It is worth mentioning that this precautionary approach also reduces the chances to detect false negatives (type II error). All graphs were created using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Protoplast regeneration under white LEDs
Freshly isolated protoplasts showed 90 to 95% viability according to red chlorophyll autofluorescence. They also had no cell wall, as shown by the absence of a blue fluorescent signal after calcofluor white staining. After 3 hours of culture, cell wall formation started as blue fluorescent spots (positive staining) on the protoplast surface (Fig. 2A). These spots grew and connected each other forming an extensive area of cell wall deposition after 16 h of culture (Fig. 2B) The entire surface of the protoplast was covered by a uniform cell wall after 2 to 6 d of culture (Fig. 2C).

Regeneration of Undaria pinnatifida gametophyte-isolated protoplasts under white light-emitting diode. (A) Fluorescence image of cell wall formation from protoplast initial stage after 3 h of culture. (B) Fluorescence image of cell wall formation after 16 h of culture. (C) Fluorescence image of cell wall formation after 48 h of culture. (D) First asymmetric cell division (arrow) in the female gametophyte. (E) Branched filament (female gametophyte) after 15 d in culture. (F) Regenerated female gametophyte producing oogonia (arrow) after 22 d in culture. (G) First asymmetric cell division (arrow) in the male gametophyte. (H) Multiple rhizoid-like protrusions (male gametophyte) after 18 d in culture. (I) Regenerated male gametophyte producing spermatangia (arrow) after 26 d in culture. Areas showing bright blue fluorescence indicate cellulose deposition. The red autofluorescence of the chlorophyll reveals areas without the cell wall. Scale bars represent: A–C & G, 10 μm; D, 20 μm; E & F, 50 μm; H, 40 μm; I, 100 μm.
Protoplast regeneration started as a single bud on one pole before the first asymmetric cell division (Fig. 2D & G). Symmetric cell division was also observed among the regenerated protoplasts but at lower percentages (14–20%). After 15 d in culture, most regenerated protoplasts isolated from female gametophytes followed unipolar germination (81%). The 15-day-old female gametophytes were visible as uniseriate BFs (Fig. 2E). New thalli were distinguished after 22 d of culture. Protoplasts isolated from male gametophytes produced MRLPs after 18 d in culture (Fig. 2H). New thalli were visible after 26 d of culture. Both regenerated gametophytes developed morphologically normal reproductive tissues (Fig. 2F & I).
Comparison of the effects of different LEDs on protoplasts isolated from female gametophytes
Overall, all analyzed LED conditions induced protoplast cell divisions. In the early stages, LEDs affected the percentage of dividing cells (p < 0.001, PRE = 0.133). Moreover, blue and RB LEDs significantly increased these values by 1.5 and 1.7 times, respectively, compared with the control (Fig. 3A). LED treatments influenced the number of asymmetric cells (p < 0.001, PRE = 0.088). More asymmetric cells formed under the blue and RB conditions; however, they represented a small increase (1.1–1.2 times) compared with the control (Fig. 3B). In the late stages, LED treatments did not affect the percentage of BFs (p < 0.01) (Fig. 3C), but RB LEDs promoted a significant increase in the final gametophyte area by three times compared with white LEDs (p < 0.001, ω2 = 0.78) (Fig. 3D). LEDs also affected the final gametophyte morphology. Under red LEDs, gametophytes showed longer filaments and more branches than those under the blue, which only presented one or none. The RB treatment produced gametophytes with longer filaments and more branches than those under the blue treatment. They were also slightly shorter than gametophytes under the red condition. Gametophytes under green LEDs showed a morphology resembling white LED conditions (Fig. 5A). Chl a, Chl c, and Fx contents were not affected by the treatments (p > 0.01) (Fig. 6A–C).

Effects of light-emitting diodes (LEDs) on early (A & B) and late (C & D) stages of regeneration from female gametophytes of Undaria pinnatifida. RB represents dichromatic LEDs (red–blue at 1 : 2 ratio). BFs represent branched filaments in (C). Independent data points and averages (horizontal lines) are shown (n ≥ 5) in (A), (C), and (D). Bars in (B) represent averages (n = 6). Error bars indicate 95% confidence intervals. Asterisks (*) and double asterisks (**) depict significant differences between treatments and control (white) at p < 0.01 and p < 0.001, respectively. Different letters indicate significant differences (p < 0.01) between treatments that showed higher values than control.
Comparison of the final morphology of regenerated Undaria pinnatifida gametophytes cultured for 22 days (female) (A) and 26 days (male) (B) under different light-emitting diode (LED) conditions. RB represents dichromatic LEDs (red–blue at 1 : 2 ratio). Scale bars represent: A & B, 100 μm.

Chlorophyll a (Chl a), chlorophyll c (Chl c), and fucoxanthin (Fx) concentrations in regenerated Undaria pinnatifida female (A–C) and male (D–F) gametophytes after exposure to different light-emitting diode conditions. RB represents dichromatic light-emitting diodes (red–blue at 1 : 2 ratio). Independent data points and averages (horizontal lines) are shown (n ≥ 3). Error bars indicate 95% confidence intervals. Asterisks (*) and double asterisks (**) show significant differences between treatments and control (white) at p < 0.01 and p < 0.001, respectively.
Comparison of the effects of different LEDs on protoplasts isolated from male gametophytes
All LED conditions allowed protoplast cell divisions. In the early stages, LEDs affected the percentage of dividing cells (p < 0.001, PRE = 0.255). Red, blue, and RB LEDs significantly increased these values by 2–2.5 times, respectively, compared with the control (Fig. 4A). LED treatments also influenced the number of asymmetric cells (p < 0.001, PRE = 0.041). More asymmetric cells were formed under the green condition; however, this represented a small increase (1.1 times) compared with the control (Fig. 4B). In the late stages, only RB LEDs increased the percentage of MRLP (p < 0.001; PRE = 0.138) and final gametophyte area (p < 0.001, ω2 = 0.87) by 1.2 and 3.6 times, respectively, compared with white LEDs (Fig. 4C & D). LEDs also affected the final gametophyte morphology. Under red LEDs, gametophytes showed longer filaments with less profuse branching than those under the blue. Gametophytes grown under the RB treatment had an “intermediate” morphology. They had longer filaments than the gametophytes under the blue and more profuse branching than those under the red treatment. Gametophytes under green LEDs showed a morphology resembling white and red conditions (Fig. 5B). The Chl a content was not affected by LED treatments (p = 0.293). The Chl c content dropped under all conditions compared with the white (p < 0.001, ω2 = 0.46), whereas the Fx content decreased under blue, RB, and green conditions compared with the control (p = 0.001, ω2 = 0.48) (Fig. 6D–F).

Effects of light-emitting diodes (LEDs) on early (A & B) and late (C & D) stages of protoplast regeneration from male gametophytes of Undaria pinnatifida. RB represents dichromatic LEDs (red–blue at 1 : 2 ratio). MRLP represents multiple rhizoid-like protrusions in (C). Independent data points and averages (horizontal lines) are shown (n ≥ 4) in (A), (C), and (D). Bars in (B) represent averages (n ≥ 4). Error bars indicate 95% confidence intervals. Asterisks (*) and double asterisks (**) mark significant differences between treatments and control (white) at p < 0.01 and p < 0.001, respectively. Different letters indicate significant differences (p < 0.01) between treatments that showed higher values than control.
Comparison of the effects of different LED light intensities on protoplast regeneration
RB LEDs were chosen for testing different light intensities on protoplast regeneration because they improved it in both protoplast types. In the early stages, the percentage of dividing cells was higher under 40 and 60 μmol photons m−2 s−1 than at 20 or 80 μmol photons m−2 s−1 for both gametophytes (p < 0.001; PRE [female] = 0.134; PRE [male] = 0.236) (Fig. 7A & D). In the late stages, the percentage of BFs increased at 80 μmol photons m−2 s−1 compared with the other light intensities (p = 0.002, PRE = 0.118) (Fig. 7B), while the percentage of MLRPs was higher at 40 and 60 μmol photons m−2 s−1 than at 20 or 80 μmol photons m−2 s−1 (p < 0.001, PRE = 0.814) (Fig. 7E). The final female gametophyte area peaked at 80 μmol photons m−2 s−1 (p = 0.002, ω2 = 0.46), representing 1.8 times increase compared with the lowest value at 20 μmol photons m−2 s−1 (Fig. 7C). The final male gametophyte area showed the highest value at 60 μmol photons m−2 s−1 (p < 0.001, ω2 = 0.87), representing 2.9 times increase compared with the lowest value at 20 μmol photons m−2 s−1 (Fig. 7F).

Effect of different dichromatic light-emitting diode intensities (red–blue at 1 : 2 ratio) on regeneration of protoplasts isolated from Undaria pinnatifida female (A–C) and male (D–F) gametophytes. BFs represent branched filaments in (B). MRLPs represent multiple rhizoid-like protrusions in (E). Independent data points and averages (horizontal lines) are shown (n ≥ 4). Error bars indicate 95% confidence intervals. Different letters indicate significant differences at p < 0.01.
DISCUSSION
Protoplast regeneration in Laminariales usually exhibits symmetric or asymmetric cell division, followed by uni- or multipolar development (Benet et al. 1997). Our results show that most protoplasts from Undaria pinnatifida gametophytes undergo simple regeneration with the first asymmetric cell division. Although symmetric cells were also present, their percentage was low. Gametophytes differed in their regeneration pathways. Female protoplasts regenerated through unipolar development, whereas the male through multipolar. These differences were not pointed out in previous studies (Zha and Kloareg 1996, Benet et al. 1997).
Visible light has been tested to enhance the growth of different seaweed species. In brown algae, Xu et al. (2005) and Miki et al. (2017) showed that blue LEDs improved growth in U. pinnatifida female gametophytes and Sargassum horneri germlines and immature stages. Moreover, Asensi et al. (2001) reported that blue light favors sporophyte regeneration from callus-like cell suspension cultures. Although our experiments also showed a positive effect of blue LEDs on protoplast regeneration, especially in the early stages, dichromatic LEDs were more effective on protoplast regeneration than white or the pure primary color LEDs. This effect was also evident in the final gametophyte morphology. A similar response was observed in the red alga Gracilaria tikvahiae, which showed higher growth under dichromatic (red + green or green + blue) or trichromatic (red + green + blue) LEDs versus the blue (Kim et al. 2015).
Our results contrast with the morphologies reported by Sato et al. (2020) in U. pinnatifida female gametophytes. For instance, they showed that gametophytes under red LED presented short filaments and few branches, a morphology similar to the one obtained in our blue LED treatment. These differences might be attributed to the fact that gametophytes originated from protoplasts in our work, while Sato et al. (2020) obtained female gametophytes from zoospores. Also, in Sato’s work, gametophytes were initially cultured under white fluorescent light and then exposed to LEDs. Thalli morphology and growth in algae can be affected by changes in light color, even during short periods (Kuwano et al. 2014).
Blue light is efficiently absorbed by the accessory pigment Fx and thus has a crucial role in brown algal growth. It upregulates the genes encoding photosystem components: F-type H+-ATPase, cytochrome b6/f complex, and ferredoxin (Foster and Dring 1994, Wang et al. 2013), which stimulates photosynthesis. Blue light perception in Phaeophyceae occurs via cryptochromes and aureochromes (Takahashi et al. 2007, Deng et al. 2012, Wang et al. 2013). Interestingly, metabolism and growth in kelps may depend on the proportion of blue light illumination versus red (Wang et al. 2013), namely the increase in blue and reduction in red. This effect of the light ratio could explain our results with RB LEDs, where the red light was present at a lower proportion than the blue.
The amounts of Chl a, Chl c, and Fx should change under different light conditions because of their specific wavelength absorption patterns. Indeed, blue light enhances Chl a content in U. pinnatifida female gametophytes and the immature stage of S. horneri (Miki et al. 2017). Furthermore, white light has shown a positive effect on Chl c and carotenoid content in both male and female U. pinnatifida gametophytes (Xu et al. 2005). In our experiments, LED treatments did not affect the pigment composition of regenerated female gametophytes. The lack of variation in pigment composition has been previously reported in the marine microalga Tetraselmis suecica and lettuce Lactuca sativa var. capitata under different color LEDs. This finding may be because the light intensity has reached a minimal value allowing sufficient synthesis and activity of photosynthetic pigments and electron carriers (Lin et al. 2013, Abiusi et al. 2014). Another explanation is that the pigment changes were transitory and thus not registered in the final pigment content. We also showed that Chl c and Fx concentrations were reduced under all light treatments compared with the control, but only in regenerated male gametophytes. The differences in pigment composition between male and female gametophytes can arise from different pigment and protein complexes (Xu et al. 2005).
Studies on Ulva and Pyropia protoplast cultures showed light intensity has little effect on regeneration (Reddy and Fujita 1991, Gall et al. 1993). In our experiments, whole plants regenerated under all RB LED light intensities, confirming the stronger resistance of protoplasts isolated from Laminariales gametophytes towards high illumination (Benet et al. 1997). Although this effect was similar for both gametophytes in the early stages, the optimum light intensity varied in the late stages. Protoplasts from female gametophytes showed improved regeneration at 80 μmol photons m−2 s−1, while their male counterparts showed better results at lower intensities (40–60 μmol photons m−2 s−1). Additionally, the light intensity had a higher size effect on male gametophytes than on the female. Ideal light intensities for U. pinnatifida gametophyte growth are between 40 and 60 μmol photons m−2 s−1 (Kim and Nam 1997, Choi et al. 2005, Xu et al. 2005, Deng et al. 2009), which is similar to our findings for male gametophytes. Xu et al. (2005) found no difference in the optimal light intensities for male and female U. pinnatifida gametophytes based on the photosynthetic rate. Our results, nonetheless, showed that optimal light intensities depended on gametophyte sex. Destombe and Oppliger (2011) found that sex strongly influenced the growth rate of the Laminaria digitata gametophytes due to different reproductive strategies. It should also be noted that previous studies have not assessed growth by calculating the gametophyte area and that all used white or monochromatic light.
In conclusion, this study is the first to report on the effect of LEDs on protoplast regeneration in multicellular marine algae. Our results suggest that dichromatic LEDs (red–blue at 1 : 2 ratio) are the most favorable for cell division and elongation of U. pinnatifida gametophyte-isolated protoplasts. LEDs affected only Chl c and Fx content in male gametophytes. Under the dichromatic light, the optimal light intensity for female gametophytes was 80 μmol photons m−2 s−1, and 40 to 60 μmol photons m−2 s−1 for the male. These results suggest that dichromatic LEDs can be used as a suitable light source for enhancing protoplast regeneration in brown algal species. Despite the well-known effects of blue and red light in kelp gametophytes, e.g., development and sexual reproduction (Sato et al. 2020, Edwards 2022), this work advances the understanding of light spectral quality in kelp gametophytes by showing, for the first time, the importance of simultaneous red and blue illumination in gametophyte development. Further studies with different light ratios are needed to clarify the effect of dichromatic light on brown seaweed growth and development.
ACKNOWLEDGEMENTS
This study was supported by research fund from Chosun University, 2021 to T. O. Cho.
Abbreviations
BF
branched filament
Fx
fucoxanthin
LED
light-emitting diode
MRLP
multiple rhizoid-like protrusions
PRE
proportional reduction of error
RB
mixed red and blue
Notes
The authors declare that they have no potential conflicts of interest.