ABSTRACTThe genus Coolia A. Meunier 1919 has a global distribution and is a common member of epiphytic dinoflagellate assemblages in neritic ecosystems. Coolia monotis is the type species of the genus and was the only known species for 76 years. Over the past few decades, molecular characterization has unveiled two species complexes that group morphologically very similar species, so their limits are often unclear. To provide new knowledge on the biogeography and species composition of the genus Coolia, 16 strains were isolated from Bahía de La Paz, Gulf of California. The species were identified by applying morphological and molecular approaches. The morphometric characteristics of all isolated Coolia species were consistent with the original taxa descriptions. Phylogenetic analyses (large subunit [LSU] rDNA D1 / D2 and internal transcribed spacer [ITS] 1 / 5.8S / ITS2) revealed a species assemblage comprising Coolia malayensis, C. palmyrensis, C. tropicalis, and the C. cf. canariensis lineage. This is the first report of Coolia palmyrensis and C. cf. canariensis in Mexico and C. tropicalis in the Gulf of California. Our results strengthen the biogeographical understanding of these potentially harmful epiphytic dinoflagellate species.
INTRODUCTIONEpibenthic dinoflagellates are globally distributed in intertidal and estuarine ecosystems, particularly in shallow, sandy, and well-illuminated environments (Fraga et al. 2012). They inhabit the interstitial spaces of marine sediments or live epiphytically on macroalgae and seagrasses (Hoppenrath et al. 2014). The epiphytic community assemblage is usually comprised (in order of prevalence) of species belonging to the genera Prorocentrum Ehrenberg 1834, Gambierdiscus Adachi & Fukuyo 1979, Ostreopsis J. Schmidt 1901, Coolia Meunier 1919, Fukuyoa Qiu, Lopes & Lin 2015, Amphidinium Claperède & Lachmann 1859, Bysmatrum M. A. Faust & K. A. Steidinger 1998, Sinophysis Nie & C. Wang 1944, and Cabra Shauna Murray & D. J. Patterson 2004, among others less prevalent genera (Hoppenrath et al. 2014, Rhodes and Smith 2018).
Epibenthic dinoflagellates produce toxins that affect human and environmental health (Bomber and Aikman 1989). Benthic harmful algal blooms (BHABs) are becoming more frequent and severe, probably due to anthropogenic influences and global climate change (Tester et al. 2020). As of today, approximately 48 taxa are recognized as harmful (Lundholm et al. 2009) and associated with ciguatera fish poisoning (CFP) (Bomber and Aikman 1989, Karafas et al. 2015, Caruana and Amzil 2018), as well as respiratory and dermatologic syndromes (Lewis et al. 2018).
Fourteen Gambierdiscus, 13 Prorocentrum, 7 Ostreopsis, and 3 Fukuyoa species have been recognized as toxic (Akselman and Fraga 2022, Hoppenrath 2022), and there is a high probability that toxins produced by some Coolia species also play a central role in harmful events (GEOHAB 2012). Also, Coolia blooms may impact the ecology through high mucilage production or lead to anoxic events (GEOHAB 2012).
The taxonomy of epibenthic dinoflagellates is currently under revision due to multiple similarities among the taxa described. This is being conducted through a morphological assessment, and new species have been discovered using molecular methods only (GEOHAB 2012). Significant progress has been made in clarifying the taxonomy of epibenthic Gonyaulacales over the past two decades. Seventeen new taxa were classified in the genera Gambierdiscus (Litaker et al. 2009, Fraga et al. 2011, 2016, Fraga and Rodríguez 2014, Nishimura et al. 2014, Smith et al. 2016, Kretzschmar et al. 2017, Rhodes et al. 2017, Jang et al. 2018), Coolia (Ten-Hage et al. 2000, Fraga et al. 2008, Leaw et al. 2010, Karafas et al. 2015, David et al. 2020) and Ostreopsis (Accoroni et al. 2016, Verma et al. 2016).
There are eight currently accepted species in the genus Coolia (Guiry and Guiry 2021). Coolia monotis was the first taxon described (Meunier 1919) and has been considered predominant. However, since the beginning of this century, molecular sequencing and phylogenetic analyses have revealed that this species actually comprises a species complex, and its distribution is not as cosmopolitan as previously thought (Momigliano et al. 2013). The description of Coolia areolata L. Ten-Hage, J. Turquet, J. P. Quod & Couté 2000 (Ten-Hage et al. 2000) was based only on morphometric and tabular examinations (Ten-Hage et al. 2000), without being genetically characterized so far. Coolia guanchica H. David, Laza-Martínez, F. Rodríguez & S. Fraga (David et al. 2020) is the most recently described taxon; so far, C. tropicalis M. A. Faust (Faust 1995) is the only species recognized as toxic (Holmes et al. 1995, Tibiriçá et al. 2020) in the Taxonomic Reference List of Harmful Microalgae (Akselman and Fraga 2022). Nevertheless, C. malayensis Leaw, P. -T. Lim & Usup 2010 (Leaw et al. 2010), C. tropicalis, C. palmyrensis Karafas, Tomas & York 2015 (Karafas et al. 2015), and C. santacroce Karafas, Tomas & York 2015 (Karafas et al. 2015) have been reported to be toxic, with biotoxin production confirmed through cytotoxicity bioassays, hemolytic assays, and chemical analyses (Holmes et al. 1995, Karafas et al. 2015, Wakeman et al. 2015, Leung et al. 2017, Tibiriçá et al. 2020).
Currently, the species in the genus Coolia have been grouped into two species complexes: the “Coolia monotis” complex, including C. monotis C. malayensis, C. palmyrensis, and C. santacroce; and the “C. canariensis” complex, comprising C. canariensis S. Fraga 2008 (Fraga et al. 2008), C. tropicalis, C. areolata, and C. cf. canariensis (Karafas et al. 2015). Species are differentiated primarily based on the shape of the epitheca; the size of some hypotheca plates and the ornamentation of the theca also distinguish species from one another. The size, shape, and position of apical pore plate (Po) and plates 1′, 2′, 4′, 6″, 7″, and 3″′ are traits used for species differentiation. The position and shape of plate 1′ differentiate at the species complex level, the size of plate 3″′ differentiate between very similar species, such as C. malayensis and C. monotis, and the shape of plate 4′ and the size of plate 6′ separates C. tropicalis from C. malayensis (de Queiroz-Mendes et al. 2019). Additional morphometric and tabular characteristics used to differentiate C. malayensis from C. monotis are cell size, plate 3′ shape, and Po length (Mohammad-Noor et al. 2013). C. malayensis is generally smaller than C. monotis (Leaw et al. 2016), although there is some size range overlap, and C. malayensis is usually narrower. Plate 3′ is pentagonal in C. monotis and quadrangular or pentagonal in C. malayensis (Mohammad-Noor et al. 2013). In C. malayensis, Po length is shorter and its postcingular plate 3″′ is the largest in the hypotheca; in C. monotis, plate 3″′ can be either equal (as shown in Aligizaki and Nikolaidis 2006, David et al. 2014, Leaw et al. 2016, Lewis et al. 2018) or larger (as indicated in Meunier 1919, Penna et al. 2005, Dolapsakis et al. 2006, Laza-Martínez et al. 2011) than plate 4″′. Both size variations in postcingular plates 3″′ and 4″′ were observed by Abdennadher et al. (2021) in C. monotis strains sampled in Southern Tunisia (Mediterranean Sea).
In Mexico, research on dinoflagellates has focused mainly on planktonic species from the Mexican Pacific, and taxonomic studies are limited to the assessment of morphological traits (Okolodkov and Gárate-Lizárraga 2006). A few studies on species composition and distribution of benthic dinoflagellates have been conducted in North Atlantic coastal ecosystems in the southern Gulf of Mexico and the Yucatán Peninsula (Okolodkov et al. 2007, Almazán-Becerril et al. 2015).
Currently, Coolia cf. areolata (Irola-Sansores et al. 2018), C. malayensis (Sepúlveda-Villarraga 2017, Ramos-Santiago 2021), C. monotis (Okolodkov and Gárate-Lizárraga 2006, Okolodkov et al. 2007, Almazán-Becerril et al. 2012, Gárate-Lizárraga et al. 2019), and C. tropicalis (Almazán-Becerril et al. 2015) have been found and characterized in Mexican ecosystems. In the Gulf of Mexico and the Mexican Caribbean, Coolia species display no particular affinity for any macrophyte substrate. In the Veracruz reef zone, C. monotis has been found associated with the seagrass Thalassia testudinum K. D. Koenig 1805, which is the most abundant and permanent host species (Okolodkov et al. 2007). However, the seagrasses Halodule wrightii Ascherson 1868 and Syringodium filiforme Kützing 1860 are also dominant species in the Gulf of Mexico, along with mainly green macroalgal species, such as Caulerpa ashmeadii Harvey 1858, C. paspaloides (Bory) Greville 1030, C. prolifera (Forsskål) J. V. Lamouroux 1809, Halimeda incrassata (J. Ellis) J. V. Lamouroux 1816, and Udotea flabellum (J. Ellis & Solander) M. Howe 1904 (Okolodkov et al. 2007). In the Mexican Caribbean, Coolia species have been found associated with Amphiroa J. V. Lamouroux, 1812 and Dictyota, J. V. Lamouroux, 1809; particularly, C. tropicalis is a very conspicuous species on a variety of macroalgae along the coast (Almazán-Becerril et al. 2015). In Bahía de La Paz, southern Gulf of California, Coolia malayensis was isolated from Sargassum horridum Setchell & N. L. Gardner 1924 (Sepúlveda-Villarraga 2017). As far we know, there are no records of harmful Coolia blooms in Mexico, and the toxicity of this genus has not been investigated.
We isolated several Coolia strains from Bahía de La Paz in the southwestern Gulf of California, which yielded new information on the biogeography and biodiversity of the genus Coolia in North Pacific coastal ecosystems in Mexico. We used morphological and molecular techniques (DNA sequencing and phylogenetics) to determine the taxonomic identity (species) of these strains.
MATERIALS AND METHODSSite descriptionBahía de La Paz is located on the Gulf of California western coast (24°28′27″ N, 110°33′2″ W), delimited by Isla San José to the north (24°58′23″ N, 110°36′52″ W), the Baja California Peninsula to the west and south, and Isla Espiritu Santo and Isla La Partida to the east (Fig. 1). Isla San José is the largest island off the east coast of Baja California Sur; its southwestern end (24°52′32″ N, 110°33′30″ W) is characterized by extensive beaches and a small lagoon bordered by mangrove forest and a narrow sand bar. Macroalgae assemblages in Bahía de La Paz usually comprise the phyla Rhodophyta, Ochrophyta-Phaeophyceae, and Chlorophyta, with the families Rhodomelaceae and Ceramiaceae being the most abundant (Piñón-Gimate et al. 2020).
Collection and preliminary handling of macroalgae samplesMacroalgae were collected from 5 selected sites in Bahía de La Paz, both by scuba diving or snorkeling and by hand (Table 1, Fig. 1). At site 1, the macroalgae sample was directly collected from a dock at 0.5 m depth. In Balandra beach (sites 3 and 4), macroalgae samples were collected 5–15 m from the coastline at 1.5 m depth, and in Isla San José lagoon at 5 m depth (site 5). Macroalgae were sampled individually by detaching from the substrate and placed separately in a resealable plastic bag or flask. These plastic bags or flasks were filled with the surrounding seawater, sealed tightly, and transported in a cooler to the laboratory for processing.
Once in the laboratory, macroalgae samples were processed following the protocol by Reguera et al. (2011) as follows: samples were vigorously shaken (2 min) to detach the cells. Macroalgae were removed, and the water suspension was filtered and washed with filtered seawater (TCLP glass fiber filters, 0.7 μm; Pall Laboratory, Port Washington, NY, USA) through overlapping sieves (250, 150, and 20 μm) to remove large particles and concentrate the epiphytic dinoflagellates (20–150 μm fraction). Finally, the cell concentrates were transferred to sterile plastic tubes (50 mL).
Cell isolation and strain establishment
Coolia strains were isolated from macroalgae of the phyla Chlorophyta and Rhodophyta; besides, some samples were collected with a plankton net (20 μm, vertical trawls) starting from 20 and 7 m depth at sites 2 and 5, respectively. Cells were isolated with the micropipette technique under an inverted microscope (Axiovert 100; Carl Zeiss USA, Thornwood, NY, USA). Isolated cells were washed three times with sterile seawater and individually transferred to 24-well tissue culture plates (Falcon, Corning, NY, USA), previously filled with either GSe (Doblin et al. 1999), L1 (Guillard and Hargraves 1993), or f/2 (Guillard and Ryther 1962) culture media. The latter was modified by adding H2SeO3 (10−8 M) and reducing the concentration of CuSO4 to 10−8 M (Anderson et al. 1984) because the absence of selenium can limit dinoflagellates growth (Mitrovic et al. 2004) while copper can inhibit it (Herzi et al. 2013). The seawater salinity used to prepare culture media varied between 37 and 39 psu. Considering the prevailing in-vitro temperature range during sample collection, cultures were grown at 20 ± 2 or 25 ± 2°C, with 40 μmol m−2 s−1 photon irradiance (12 : 12 h Light : Dark), which are the standard conditions defined for the Marine Dinoflagellate Collection (CODIMAR, for its acronym in Spanish). Clonal cultures were deposited in CODIMAR: https://www.cibnor.gob.mx/investigacion/colecciones-biologicas/codimar.
Strain identificationMicroscopyTheca examination and morphometric measurements of specimens of each strain were carried out using a phase-contrast microscope (BX41TF; Olympus, Tokyo, Japan) equipped with a U-ECA 2 magnification changer and an ocular micrometer. Photographic records were captured with a digital camera (CoolSNAP-Pro; Media Cybernetics, Bethesda, MD, USA) and an image processing software (Image-Pro Plus 4.1 for Windows; Media Cybernetics, Silver Spring, MD, USA). Cells were observed live or fixed with 4% (v/v) formaldehyde or acidic Lugol’s solution. All morphometric measurements of each strain were made with fixed samples, and a variable number from 6 to 25 specimens was measured with the ocular micrometer to determine cell length, cell width, and dorsoventral (DV) depth. For the plate pattern analysis, cells were dissected by pressing the coverslip with a needle to squash the theca, adding sodium hypochlorite solution as needed. The kofoidian plate tabulation was used for plate designation (Kofoid 1909). Specific theca measurements, such as Po length and plate 7″ length (L) : width (W) ratio, were made using micrographs captured with the magnification marker and measurement tools of the image analysis software. To test for significant differences between strains, cell size measurements and Po length of each species were analyzed using a one-way ANOVA.
Cells were processed for scanning electron microscopy (SEM). First, cells were washed with filtered seawater (0.2 μm, Supor 200 Membrane Disc Filters 60301; Pall Laboratory) supplemented with antibiotics and then treated with 10% or 15% Triton X-100 solution (X100; Sigma-Aldrich, St. Louis, MO, USA) to remove external membranes. Cell fixation was accomplished with 2% (v/v) glutaraldehyde solution (16020; Electron Microscopy Sciences, Delray Beach, FL, USA) for 1 h, followed by post-fixation with 1% (w/v) osmium tetroxide (O5500; Sigma-Aldrich) overnight at 4°C. Then, cells were washed three times to remove fixatives, first with a 1 : 1 solution of filtered seawater (0.22 μm) and distilled water, then three times with distilled water. Finally, cells were collected on polycarbonate filters (8 μm, Nuclepore 110414; Whatman, Maidstone, Kent, UK) and dehydrated by immersion in ethanol of progressive concentration (277649; Sigma-Aldrich) from 30 to 100% in eight steps. Filters were critical-point dried with CO2 (Samdri PVT3B; Tousimis Research, Rockville, MD, USA), glued onto stubs, and sputter-coated with palladium (vacuum desk II; Denton Vacuum, Moorestown, NJ, USA). An additional batch of samples was fixed with 2% (v/v) glutaraldehyde only and dehydrated as described above but dried with HMDS (44091; Sigma-Aldrich), placing the specimens on polycarbonate filters (3 μm, Nuclepore 110412; Whatman). Filters were examined under two scanning electron microscopes (S-3000N; Hitachi, Tokyo, Japan and JSM 6360 LV; Jeol Ltd., Tokyo, Japan).
DNA extraction, PCR amplification, and sequencingDNA was extracted from 25-mL cultures in the logarithmic growth phase using the FastDNA SPIN Kit for Soil (Catalog # 6560-200; MP Biomedicals, Solon, OH, USA). PCR amplifications were performed in a 50-μL reaction volume using a GoTaq Flexi DNA Polymerase kit according to the manufacturer’s protocol (Promega, Madison, WI, USA). The large subunit (LSU) rDNA D1 / D2 region was amplified using D1R (5′-ACCCGCTGAATTTAAGCATA-3′) and D2C (5′-CCTTGGTCCGTGTTTCAAGA-3′) primers (Scholin et al. 1994) and the internal transcribed spacer (ITS) 1–5.8S-ITS2 region of the rDNA using the ITSA (5′-CCTCGTAACAAGGHTCCGTAGGT-3′) and ITSB (5′-CAGATGCTTAARTTCAGCRGG-3′) primers (Adachi et al. 1996). Thermocycler conditions with D1R and D2C were as follows: initial denaturation at 95°C for 1 min, followed by 35 cycles of 95°C for 30 s, 45°C for 30 s, and 72°C for 1 min 45 s, with a final extension period at 72°C for 5 min. Thermocycler conditions with ITSA and ITSB were as follows: initial denaturation at 94°C for 3.5 min followed by 35 cycles at 94°C for 50 s, 47°C for 60 s, and 72°C for 80 s, with a final extension period at 72°C for 10 min. The amplification products were purified and Sanger sequenced (Macrogen, Seoul, Korea), both forward and reverse.
LSU rDNA and ITS sequence analysis and phylogenetic reconstructionThe LSU rRNA gene and ITS1-5.8S-ITS2 region sequences obtained in this study were aligned with Coolia sequences downloaded from the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) (Benson et al. 2013). Ostreopsis cf. ovata was selected as the outgroup. Sequences were aligned online using MAFFT v7.110 (Katoh and Standley 2013) with the default setting values. Complete alignments were then checked using BioEdit v7.0.5 (Hall 1999). The final alignment consisted of 935 (LSU, 70 taxa) and 539 (ITS, 45 taxa) base pairs, including the introduced gaps. The best molecular evolution model (GTR + G) was selected by jModelTest (Posada 2008) based on the Akaike Information Criterion for both Bayesian inference and maximum likelihood (ML) analyses. Bayesian inference was performed using MrBayes 3.2 (Ronquist and Huelsenbeck 2003). Four Markov chain Monte Carlo chains were run for 2,000,000 generations with sampling every 1,000 generations. The first 10% of burn-in trees were discarded. The Bayesian posterior probability of each clade was determined. ML analyses were conducted online (Boc et al. 2012) using RaxML v7.2.6 (Stamatakis 2006). Bootstrap support was assessed with 1,000 replicates.
Pairwise genetic distances based on LSU rDNA (D1–D2) and ITS region sequences were calculated using the PAUP*4b10 software (Swofford 2002).
RESULTSWe isolated 16 Coolia strains from the macroalgae Ceramium sp., Halimeda discoidea Decaisne 1842, Laurencia sp., Spyridia filamentosa (Wulfen) Harvey 1833, and Ulva sp. (Table 1). From these, we identified the following species using morphological and molecular techniques: Coolia tropicalis (2 strains), C. palmyrensis (2 strains), C. malayensis (8 strains), and the genetic lineage C. cf. canariensis (4 strains) (Table 2). Coolia cf. canariensis and C. palmyrensis had not been documented previously in Mexican coastal waters, and C. tropicalis was found in the Gulf of California for the first time. Coolia species, particularly at Isla San José, shared their habitat with other epiphytic dinoflagellates of the genera Amphidinium, Fukuyoa, Gambierdiscus, Ostreopsis, and Prorocentrum.
Species descriptionsCoolia malayensis Leaw, P. -T. Lim & Usup (Fig. 2)Cell shape round; size variation across strains: 23–44 μm long, 23–39 μm wide, and 29–39 μm DV depth (Table 2). Epitheca slightly smaller than hypotheca (Fig. 2E). Thecal plates smooth and irregularly scattered with round pores of an average diameter of 0.31 ± 0.03 μm (Table 2, Fig. 2A, E & G), and 3–6 poroids inside each pore (Fig. 2I). Po 5–11 μm long, narrow (Table 2, Fig. 2B, E & F), and off-centered, neighboring the apical plate 2′ (Fig. 2E & F). First apical plate (1′) narrow and oblong; plate 2′ narrow, elongated, and in direct contact with Po along its left and dorsal sides (Fig. 2B, E & F). Plate 3′ pentagonal (Table 2, Fig. 2B & E), contacting plates 1′, Po, 2′, 4″, 5″, and 6″ (Fig. 2E & F). Precingular 6″ is the largest plate in the epitheca (Fig. 2A, B & D). Width-to-length ratio of the seventh precingular plate (7″) from 1–2 between strains (Table 2). In the hypotheca, postcingular plates 3″′ and 4″′ are the largest (Fig. 2C, G & H), the former quadrangular and the latter triangular. Plates 2″′ and 5″′ smaller; plate 1″′ is the smallest (Fig. 2C, G & H). The differences in cell length (F7, 158 = 3.2; p = 0.003), width (F7, 158 = 2.3; p = 0.03), and Po length (F7, 87 = 3.6; p = 0.002) were significant across strains, while differences in DV depth were not significant (F7, 104 = 1.3; p = 0.25).
Coolia palmyrensis Karafas, Tomas & York (Fig. 3)Cell shape round; size variation between strains: 23–31 μm long, 21–31 μm wide, and 20–26 μm DV depth (Table 2, Fig. 3A, C & F). Theca surface smooth and sparsely dotted with pores of an average diameter of 0.32 ± 0.07 μm (Table 2, Fig. 3C & F–H), and 3–6 poroids inside each pore (Fig. 3I). Po short in length (5–8 μm), slightly curved in apical view (Fig. 3D & E), and off-centered, abutting apical plate 2′ (Fig. 3G). Plate 1′ hexagonal and narrow (Fig. 3B, C & G); precingular 6″ is the largest plate in the epitheca (Fig. 3C & G). Width-to-length ratio of the seventh precingular plate (7″) from 1–2 between strains (Table 2, Fig. 3C). In the hypotheca, postcingular 3″′ is the largest plate, followed by plates 2″′ and 4″′, which are almost equal in size (Fig. 3F & H). Plate 2″′ medium-sized; 1″′ is the smallest plate (Fig. 3F & H). Antapical plate 1″′ larger than plate 2″″ and the two contact each other (Fig. 3F). These particular strains were susceptible to damage from SEM treatments, which precluded documenting all their characteristics. The differences in cell length (F1, 48 = 0.19; p = 0.89), width (F1, 48 = 1.5; p = 0.23), DV depth (F1, 33 = 0.15; p = 0.70), and Po length (F1, 25 = 1.1; p = 0.31) were not significant.
Coolia tropicalis M. A. Faust (Fig. 4)Cell shape round; size variation between strains: 31–44 μm long, 31–46 μm wide, and 36–44 μm DV depth (Table 2, Fig. 4D, F & G). Epitheca slightly smaller than hypotheca (Fig. 4D & G). Theca surface smooth and moderately covered with scattered round pores (Fig. 4B, C & E) of an average diameter of 0.28 ± 0.04 μm. Po off-centerer beside apical plate 2′ (Fig. 4A, G & H), 7–11 μm in length (Table 2). Apical plate 1′ located centrally in the epitheca; it is the widest on the ventral side (Fig. 4B & D); plate 3′ pentagonal (Fig. 4A & B). Width-to-length ratio of the seventh precingular plate (7″) from 2–4 across strains (Table 2, Fig. 4E). Equatorial deep cingulum bordered by list with smooth edges (Fig. 4D & G). Sulcus deep not reaching the cell antapex (Fig. 4D & F). A narrow list partially covering the sulcus on either side (Fig. 4D), and a narrower list located on the antapical side. In the hypotheca, postcingular plates 3″′ and 4″′ equally large (Fig. 4C & F). Plates 2″′ and 5″′ smaller, and 1″′ is the smallest plate (Fig. 4C & F). Antapical plate 1″″ very small and plate 2″″ quadrangular (Fig. 4C & F). The differences in cell length (F1, 48 = 1.5; p = 0.23), width (F1, 48 = 2.7; p = 0.11), DV depth (F1, 31 = 3.3; p = 0.08), and Po length (F1, 48 = 0.06; p = 0.8) were not significant.
Coolia cf. canariensis (Fig. 5)Cell shape also round; size variation across strains: 26–39 μm long and 28–39 μm wide and in DV depth (Table 2). Thecal plates with numerous scattered pores (Table 2, Fig. 5C, D, F & I) of an average diameter of 0.32 ± 0.05 μm. Hypotheca with more plate pores than epitheca (Fig. 5D & E). Po length 7–13 μm (Table 2), off-centered (Fig. 5A, B & F) beside apical plate 2′, easy to observe under light microscopy (Fig. 5A–C). The hexagonal 1′ is the largest plate of the epitheca (Table 2), with a central position (Fig. 5A, C & F). Plate 2′ narrow and elongated, in direct contact with Po along its left and dorsal sides (Fig. 5C, F & G). Plate 3′ is pentagonal (Table 2) and contacts plates 1′, Po, 2′, 4″, 5″, and 6″ (Fig. 5B, C & F). The pentagonal 6″ is the largest of the precingular plates (Fig. 5C & H). Width-to-length ratio of the seventh precingular plate (7″) from 1–2 across strains (Table 2, Fig. 5E). In the hypotheca, first postcingular plate (1″′) triangular and small; 2″′, 3″′, 4″′, and 5″′ large and elongated (Fig. 5D, H & I). Antapical plate 1″′ elongated and narrow, parallel to the sulcus and bordering the posterior plate (Sp) and precingular plates 1″′ and 2″′; plate 2″″ pentagonal, contacting the Sp and postcingular plates 2″′, 3″′, 4″′, and 5″′ (Fig. 5D). Postcingular plates with concavities giving them a rough appearance (Fig. 5E & I). The differences in cell width (F3,102 = 5.7; p = 0.001), and DV depth (F3, 58 = 7.9; p = 0.0002) were significant across strains, while the differences in cell length (F3,102 = 2.3; p = 0.08), and Po (F3, 64 = 2.1; p = 0.1) were not significant.
Molecular characterizationFourteen Coolia strains were genetically characterized based on LSU rDNA (D1 / D2 region) and nine based on ITS1 / 5.8S / ITS2 sequencing (Table 3). The Bayesian phylogenetic reconstruction showed well-supported clades representing seven of the eight currently accepted Coolia species (Figs 6 & 7). Bayesian posterior probabilities and the ML bootstrap values strongly supported clade clustering, defining three groups. Strains were grouped into clades, corresponding to C. malayensis (8 strains), C. palmyrensis (1 strain), C. tropicalis (1 strain), and the C. cf. canariensis linage (4 strains). A large group was composed of clades C. malayensis, C. monotis, C. santacroce, and C. palmyrensis. In the LSU (D1–D2 region) tree, Coolia malayensis from Bahía de La Paz was grouped mainly with strains from the Indo-Pacific region; in the ITS1 / 5.8S / ITS2 tree, these strains were separated into a subgroup but shared similarities with both Atlantic and Indo-Pacific strains. Coolia palmyrensis from Isla San José was grouped with strains from Palmyra atoll (in the Central Pacific), Dominican Republic, and Brazil. A separate group was composed of three closely related species: C. guanchica, C. canariensis, and C. cf. canariensis, each establishing a well-supported monophyletic clade. Coolia cf. canariensis from Bahía de La Paz was grouped with isolates from Spain (Mediterranean Sea) and Korea (Figs 6 & 7). In a third group, C. tropicalis showed a monophyletic origin, and the strain CTJV-2 was closely related to Australian, Atlantic, and Indo-Pacific strains.
Genetic distances based on LSU (D1–D2) sequences (Table 4) showed that intraspecific values were low in C. malayensis and C. palmyrenis (<0.06) but high in C. canariensis (0.19), which was comparable to interspecific distances (0.11–0.41). Genetic distances based on ITS region sequences (Table 5) showed that intraspecific values were low in C. malayensis and C. tropicalis (<0.09) but high in C. canariensis (0.19). The latter was also comparable to interspecific distances (0.19–0.50).
DISCUSSIONThis study focused on isolating and characterizing the species composition of the genus Coolia in Bahía de La Paz, southwestern Gulf of California. Species were characterized using morphometric and molecular analyses. The 16 well-established strains comprised the species Coolia malayensis, C. palmyrensis, C. tropicalis, and the genetic lineage C. cf. canariensis.
Our results document for the first time the presence of Coolia cf. canariensis and C. palmyrensis in Mexico and also report C. tropicalis in the Gulf of California for the first time. A previous molecular characterization of a Coolia strain from Bahía de La Paz with the 28S rDNA marker also demonstrated the presence of C. malayensis (Sepúlveda-Villarraga 2017, Ramos-Santiago 2021).
Except for subtle morphometric differences, the Coolia strains found in the present study are comparable to the original taxa descriptions. Leaw et al. (2010) reported a Po length of 5 μm in C. malayensis, while our specimens measured 5–11 μm (Table 2). In the original description of C. palmyrensis, cell length (16–26 μm) and DV depth (21–30 μm) ranges were slightly wider (Karafas et al. 2015) than in our specimens (23–31 and 20–26 μm, respectively) (Table 2). Similarly, in the original description of C. tropicalis, the cell length and width ranges of 23–40 and 25–39 μm, respectively, were somewhat wider than in our specimens (31–44 μm length and 31–46 μm wide). However, DV depth, which reached 65 μm according to Faust (1995), did not exceed 44 μm (Table 2) in our specimens. The description of Coolia cf. canariensis was similar to the original C. canariensis description by Fraga et al. (2008). The distinctive features of this taxon, i.e., mean cell size (27.2–38.4 μm long and 25.6–40 μm wide), Po length (8 μm), the large size and central position of plate 1′, and plate 7″ size overlap with those of our specimens (Table 2).
The taxonomic classification of the genus Coolia is further supported by combining morphometric analysis with molecular characterization techniques. Research using both methods over the past decade has improved the delimitation of morphometric characteristics that were also supported phylogenetically. In particular, the shape and position of the first apical plate have been valuable for differentiating the two major Coolia species complexes (Karafas et al. 2015). The strains included in the present study were mainly grouped within the C. monotis and C. canariensis species complexes based on the LSU rDNA and ITS1 / 5.8S / ITS2 phylogenetic analyses; these findings are consistent with other related studies (Mohammad-Noor et al. 2013, Karafas et al. 2015, Gómez et al. 2016, Leaw et al. 2016, Lewis et al. 2018, Larsson et al. 2019, Nascimento et al. 2019, David et al. 2020, Abdennadher et al. 2021). The results of the LSU and ITS analyses strongly suggest that C. santacroce is closely related to C. monotis and that C. palmyrensis emerges earlier than both C. monotis and C. malayensis (Figs 6 & 7). This same clade arrangement was observed by Karafas et al. (2015) and Nascimento et al. (2019). In the C. canariensis complex (Figs 6 & 7), 1′ is the largest plate and has a central position in the epitheca, whereas in the C. monotis complex (Figs 6 & 7), this plate is oblong and narrow, in a central position or to the left (Karafas et al. 2015, and references therein). The shape of plate 4′ and the size of plate 6″ separate C. tropicalis from C. malayensis, while the thecal surface ornamentation differentiates C. tropicalis from C. canariensis (de Queiroz-Mendes et al. 2019). It is also worth noting that morphometric features have been proposed to discriminate closely related Coolia species, although with no phylogenetic support. These include cell size, Po size, pore size and density, presence of poroids, W : L ratio of plate 7′, L : L ratio of the APC (apical pore complex) and 2′, size and shape of plates 1′ and 3′, W : L ratio of 7″ and 6″, and size and shape of 3″′ (Fraga et al. 2008, Karafas et al. 2015, Leaw et al. 2016, de Queiroz-Mendes et al. 2019).
Based on morphometric and theca traits, we initially considered that our strains corresponded to C. canariensis. However, the phylogenetic analysis showed that these strains belong to the C. cf. canariensis lineage, specifically grouped within the phylogroup III defined by Nascimento et al. (2019) for the C. canariensis complex (Figs 6 & 7). This lineage has also been reported for strains from the Canary Islands, Mediterranean Spain, Korea, Australia, China, and Brazil (Laza-Martínez et al. 2011, Jeong et al. 2012, Momigliano et al. 2013, David et al. 2014, Leung et al. 2017, de Queiroz-Mendes et al. 2019, Nascimento et al. 2019). The lineage corresponding to the holotype (C. canariensis sensu stricto) had not been observed previously outside the Canary Islands (David et al. 2020). Coolia canariensis was described by Fraga et al. (2008) based on three strains that were split phylogenetically into two sister lineages with no morphological differences between them. In this work, we found that the C. canariensis strains were clustered in a lineage that excludes the type species (strain VGO787, AM902738), which agrees with the work based on the LSU rDNA and ITS1 / 5.8S / ITS2 phylogenetic analyses (Laza-Martínez et al. 2011, Jeong et al. 2012, Momigliano et al. 2013, David et al. 2014, 2020, Leaw et al. 2016, de Queiroz-Mendes et al. 2019, Nascimento et al. 2019). David et al. (2014) consider that the degree of separation is still insufficient to delineate clades. However, they hypothesized that there are likely two cryptic species within the C. canariensis clade; given the insufficiency of genetic data, they proposed that this should be referred to as C. cf. canariensis. Jeong et al. (2012) and Leaw et al. (2016) highlight the need to conduct additional comparative morphological and DNA sequence (in particular, ITS) analyses to assess whether or not they are cryptic species.
Based on LSU and ITS rDNA phylogenies, Nascimento et al. (2019) separate the C. canariensis complex into three phylogroups. Our results reinforce the cryptic-species hypothesis proposed by David et al. (2014) and the separation delineated by Nascimento et al. (2019) since our strains were grouped within the phylogroup III. Although our strains are morphologically very similar to the C. canariensis type species, we found differences in cell size relative to the phylogroup II. Cell size within the strain UNR-25 from Brazil was 24.9 ± 2.3 μm long, with a DV depth of 27.5 ± 2.4 μm and a Po of 7.8 ± 1.1 μm long; our strains had a size variation of 26–39 μm long, 28–39 μm wide and in DV depth, and a Po length pf 7–13 μm (Table 2). However, additional comparative morphometric, genetic, and life-cycle characterization analyses are required to determine whether or not they are cryptic species. For example, the slight anteroposterior cell compression in the strain UNR-25 found by Nascimento et al. (2019) was also identified in some of the specimens studied in the present study (Fig. 5F). This feature may be a morphological variation in cell growth, theca development, or associated with a life cycle stage.
Genetic distances based on LSU (D1–D2) and ITS region sequences appear to support that C. canariensis is a cryptic species. Genetic distances within the C. canariensis strains are similar or even greater than those among Coolia species (Tables 4 & 5).
The strain CMPV-1 (Tables 1 & 2) was morphologically (CODIMAR) (Morquecho and Reyes-Salinas 2004) and genetically (JQ638943) (Herrera-Sepúlveda et al. 2013) identified as C. monotis. In this study, however, we determined that this strain belongs to the C. cf. canariensis clade (Figs 6 & 7). The sequence JQ638943 retrieved from GenBank was previously used by David et al. (2014), Gómez et al. (2016), and Nascimento et al. (2019), who also concluded that this strain, attributed initially to C. monotis, actually belongs within the C. cf. canariensis clade. Based on our results, we suggest that this sequence in the GenBank be revised and reclassified.
It is also worth noting that our C. malayensis strains in the ITS tree were grouped separately, suggesting a certain degree of diversification. Nascimento et al. (2019) also hypothesize that this species contains a high molecular diversity. To confirm this apparent genetic diversification in the southern Gulf of California populations, it will be necessary to isolate strains from other locations in the Mexican Pacific to gather complete ITS sequences.
Our study documented four of the eight currently accepted Coolia species. This species composition has also been found in the Great Barrier Reef, Australia (Momigliano et al. 2013), China (Leung et al. 2017), and Brazil (Nascimento et al. 2019, Tibiriçá et al. 2020). These findings suggest that the genus Coolia is widespread in the Mexican Pacific, with a predominance of C. malayensis. Given the high morphological similarity among taxa, it is likely that the records of C. monotis in studies on phytoplankton species composition in the Mexican Pacific (Okolodkov and Gárate-Lizárraga 2006) correspond to C. malayensis. This is currently recognized as the most widespread species, occurring between latitudes 36° S and 34° N, while C. monotis appears to be restricted to the North Atlantic and the Mediterranean Sea (Larsson et al. 2019). As described recently, C. canariensis (Fraga et al. 2008) and C. palmyrensis (Karafas et al. 2015) still lack global distribution patterns. Together with C. areolata and C. tropicalis, these species likely occur along the tropical and subtropical Pacific coastlines. The latter two species have been reported in Isla del Coco National Park, Costa Rica (Vargas-Montero et al. 2012).
The Coolia species assemblage found in this study is potentially toxic. The production of moderately toxic bioactive compounds by C. malayensis and C. palmyrensis has been reported (Karafas et al. 2015, Wakeman et al. 2015, Li et al. 2020). However, C. tropicalis and C. malayensis are the only two species with a more accurate toxicological characterization and are recognized due to the production of cooliatoxins, which have not been chemically identified yet (Holmes et al. 1995), and two 44-methyl-gambierone isomers (Murray et al. 2020), previously limited to Gambierdiscus spp. (Tibiriçá et al. 2020). Therefore, further research is needed to characterize the toxicity of Coolia species and their influence on ecotoxicology in Bahía de La Paz and the Gulf of California.
While Coolia BHABs have not been recorded in the Gulf of California, the potential threat from these species cannot be overlooked, particularly given the projected changing global climate. Núñez-Vázquez et al. (2019) reported that CFP is the second-most prevalent form of human seafood poisoning in Mexico, after paralytic shellfish poisoning. From 1984 to 2013, 52% of the 464 ciguatera cases occurred in Baja California Sur, where this study was carried out. Interestingly, ciguatera human poisoning and ciguatoxins have been reported on the small rocky island El Pardito (Núñez-Vázquez et al. 2013), near the San José Lagoon, where several Coolia species were isolated. Coolia species can contribute to mucilage production and formation of benthic blooms leading to the development of mucilaginous aggregates, which can have serious environmental effects, including lower water quality, substrate deposits that foster the growth and transport of other harmful microorganisms, benthic fauna mortality, and contact dermatitis in humans and terrestrial fauna (Lewis et al. 2018).
In summary, this is the first comprehensive taxonomic report of the Coolia species composition in the southwest Gulf of California. The phylogenetic analysis revealed a diverse and heterogeneous Coolia species assemblage. The Coolia cf. canariensis lineage and C. palmyresis had not previously been reported along the Mexican coasts, and their geographic range now stretches to subtropical coasts of the North American Pacific. Given the great morphological similarity among taxa, the reports of C. monotis in the Mexican Pacific most likely correspond to C. malayensis. The occurrence of the toxic C. tropicalis in the Gulf of California had not been reported previously. Given the current context of global climate change, further studies are needed to delimit the species composition of the genus Coolia and assess the factors that affect their growth to determine the potential for harm and toxicity of this genus and other epiphytic dinoflagellates.
ACKNOWLEDGEMENTSJorge Angulo-Calvillo and Enrique Calvillo-Espinoza provided technical assistance in the field as divers and boat drivers. Amada Reyes-Salinas, Alejandra Mazariegos-Villarreal and Ariel Cruz-Villacorta provided technical assistance. Thelma Castellanos, Angel Carrillo and Goretty Caamal provided technical advice and infrastructure of the Molecular Microbial Ecology Laboratory. All are at CIBNOR. Yuri Okolodkov (Universidad Veracruzana) assisted during the 2016 macroalgae sampling. Laura E. Gómez-Lizárraga (SAMEB, ICMyL-UNAM) also provided technical assistance. This study was supported by the CIBNOR project 20014, and the CONACYT A1-S-37026 grant. IGL was supported by SIP-20190272 and COFAA. Taylor Morey provided English revision. María Elena Sánchez-Salazar edited the English manuscript.
Fig. 1Maps of the Gulf of California and Bahía de La Paz indicating the study area (A) and the location of the five sampling sites (B). 1, Ensenada de La Paz; 2, Isla Gaviota; 3 & 4, Balandra; 5, Isla San José. Algae 2022, 37(3): 185–204 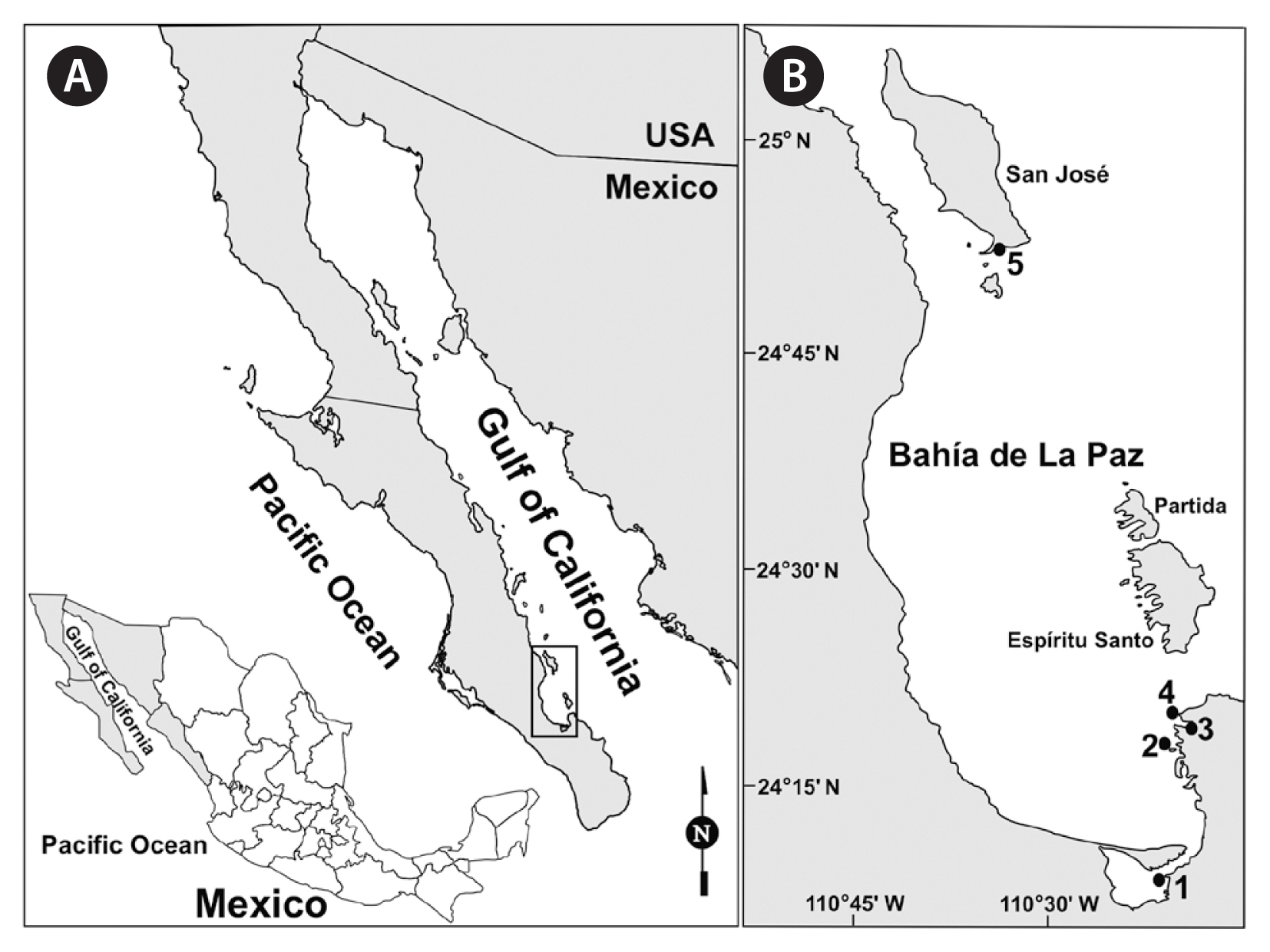
Fig. 2
Coolia malayensis. Phase-contrast light microscopy (A–C) and scanning electron microscopy (D–I). (A) Epitheca with apical 1′ and precingular 6″ plates (strain CAPV-1). (B) Epitheca with plate tabulation (strain CAPV-1). (C) Hypotheca with plate tabulation (strain CAPV-5). (D) Epitheca in lateral view with apical 1′ and precingular 6″ and 7″ plates (strain CAJV-1). (E) Theca in dorsal view with apical pore plate (Po), apical 2′ and 3′, precingular 2″, 3″, and 4″, and postcingular 2″′ and 3″′ plates (strain CAJV-1). (F) Po plate (strain CAJV-1). (G & H) Hypotheca with postcingular plates (strain CAPV-5). (I) Poroids; arrows mark the view from the inner side of the plate (strain CAPV-5). Scale bars represent: A–E, G & H, 20 μm; F, 5 μm; I, 1 μm. 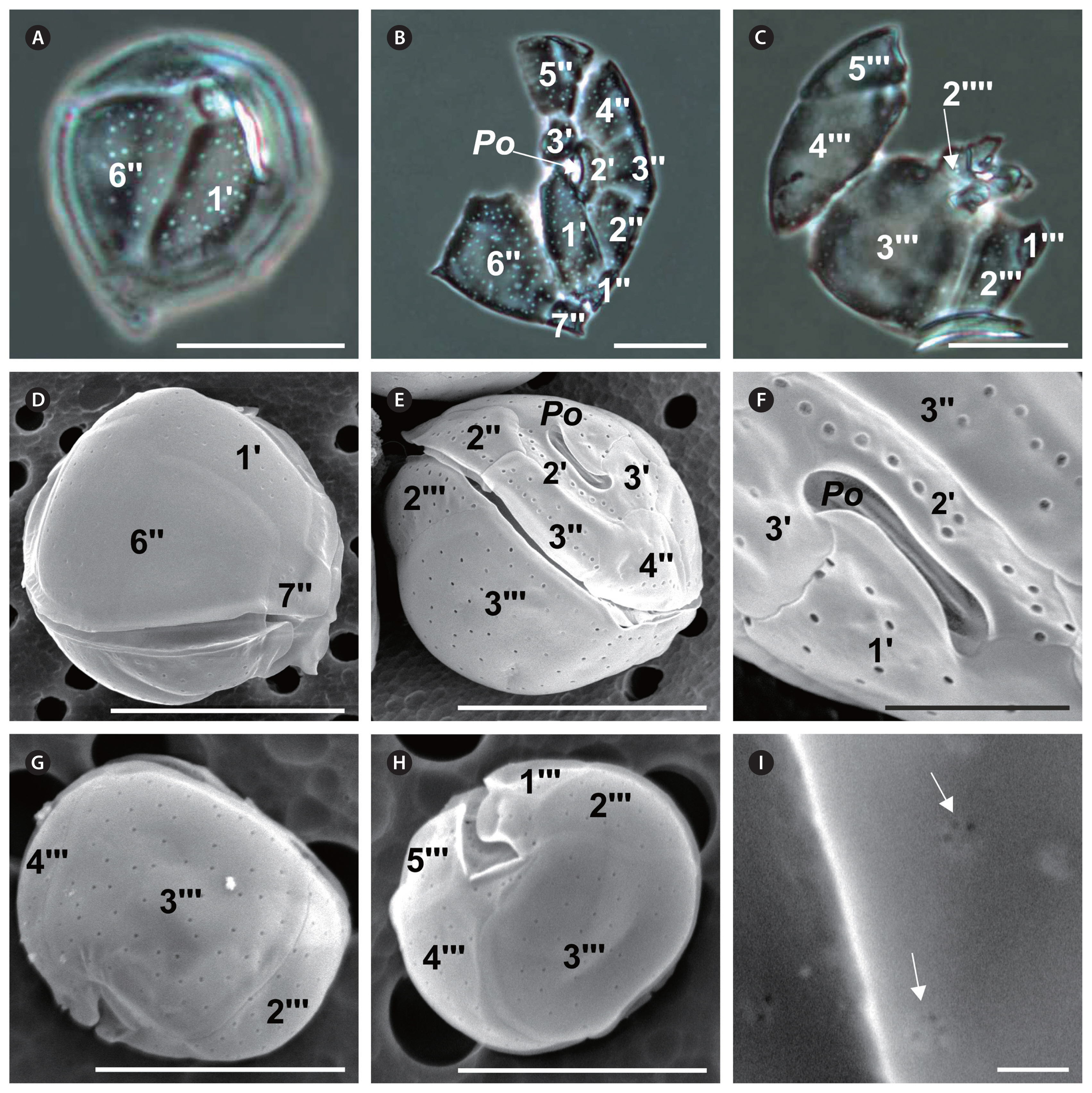
Fig. 3
Coolia palmyrensis. Phase-contrast light microscopy (A, B, D, E, G & H) and scanning electron microscopy (C, F & I). (A) Theca in ventral view showing cingulum and sulcus (strain CLJV-1). (B) Epitheca with the apical 1′ and precingular 6″ and 7″ plates (strain CLJV-1). (C) Theca in ventral view showing apical 1′ and most of the precingular plates (strain CLJV-2). (D & E) Epitheca with apical pore plate (Po) in apical (strain CLJV-2) and dorsal (strain CLJV-1) view. (F) Hypotheca with plate tabulation (strain CLJV-2). (G) Epitheca with plate tabulation (strain CLJV-2). (H) Hypotheca with plate tabulation (strain CLJV-1). (I) Zoom of pores with a dissimilar number of poroids (arrows). Scale bars represent: A–H, 20 μm; I, 1 μm. 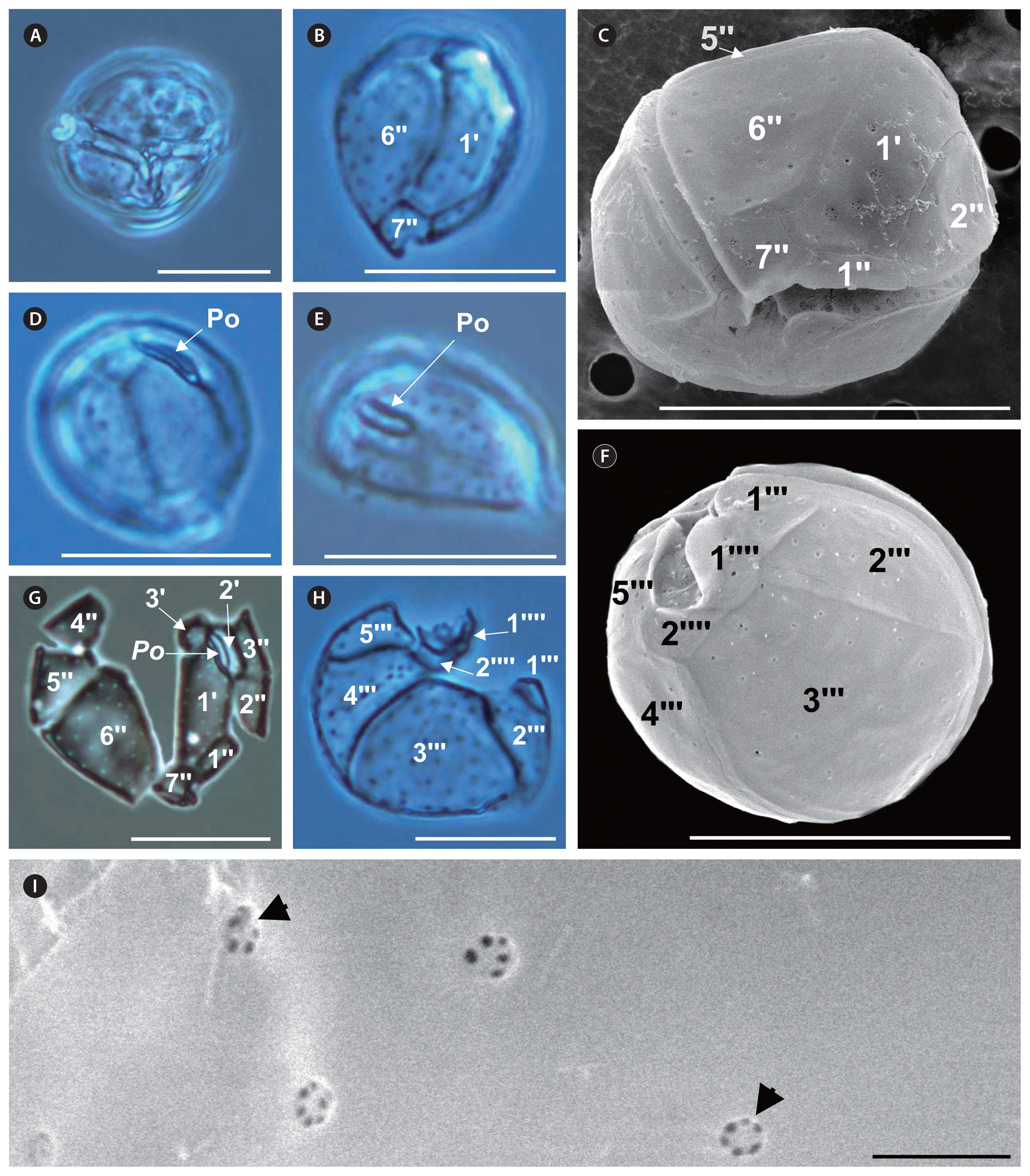
Fig. 4
Coolia tropicalis. Phase-contrast light microscopy (A–C) and scanning electron microscopy (D–H). (A) Epitheca with apical pore plate (Po) and apical plate 3′ (strain CTJV-2). (B) Epitheca with plate tabulation (strain CTJV-1). (C) Hypotheca with plate tabulation (strain CTJV-1). (D) Theca in ventral view with plate tabulation (strain CTJV-1). (E) Precingular plate 7″ (strain CTJV-1). (F) Theca in ventral-antapical view showing hypotheca tabulation (strain CTJV-1). (G) Theca in dorsal view with plate tabulation (strain CTJV-1). (H) Po plate (strain CTJV-1). Scale bars represent: A–D, F & G, 20 μm; E, 10 μm; H, 5 μm. 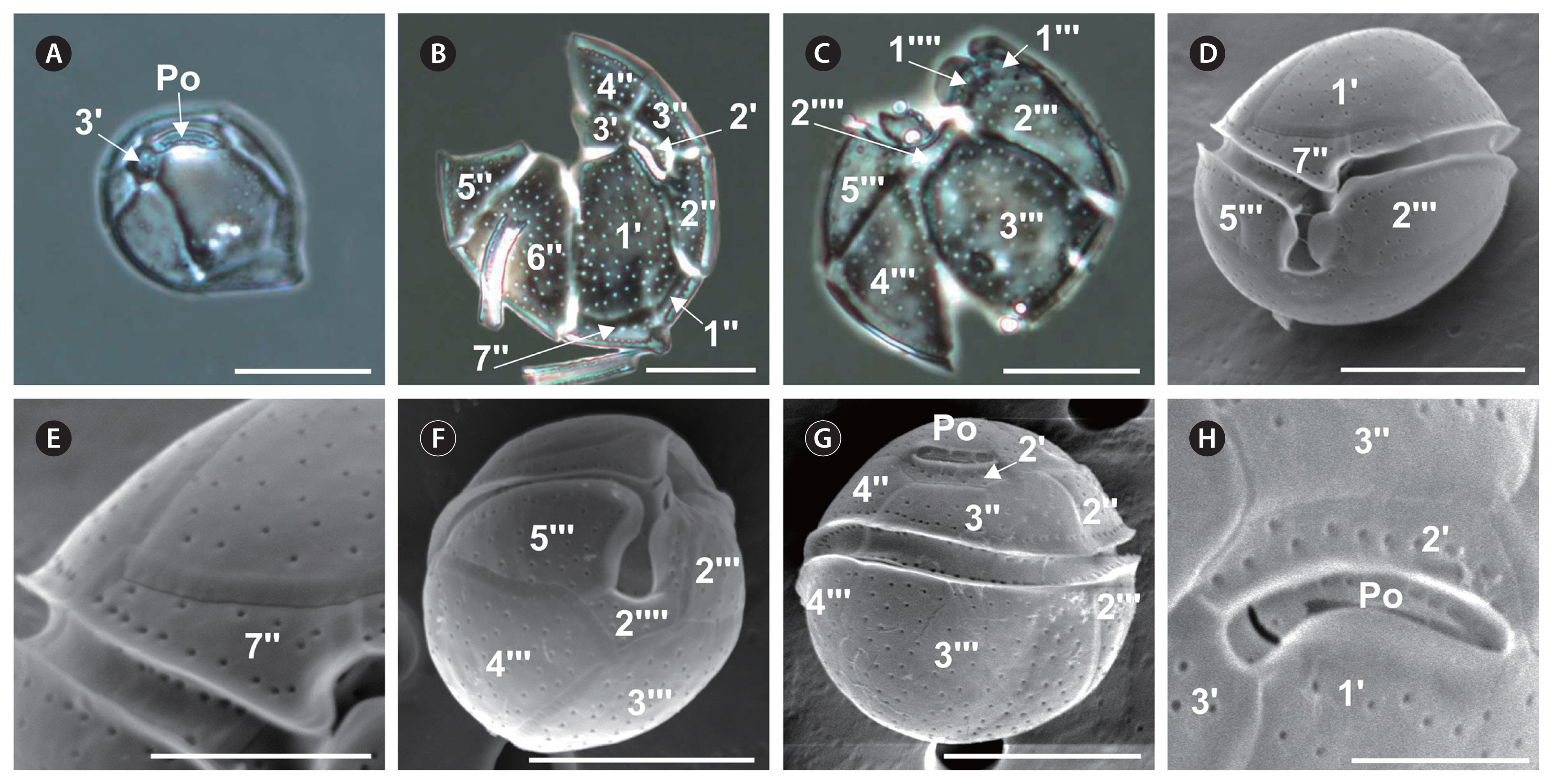
Fig. 5
Coolia cf. canariensis. Phase-contrast light microscopy (A–D), and scanning electron microscopy (E–I). (A) Epitheca with apical pore plate (Po) and apical plate 1′ (strain CCJV-1). (B) Epitheca with apical plate 3′ and Po (strain CCJV-1). (C) Epitheca with plate tabulation (strain CCJV-1). (D) Hypotheca with plate tabulation (strain CCJV-2) and posterior plate (Sp). (E) Theca in apical view showing epitheca tabulation and postcingular 5″ plate (arrow) with concavities that give a rough appearance (strain CCJV-1). (F) Epitheca showing Po and apical plates 1′–3′ (strain CCJV-1). (G) Po plate (strain CCJV-1). (H) Theca in lateral view with plate tabulation (strain CCJV-2). (I) Hypotheca with plate tabulation; concavities also apparent (strain CCJV-2). Scale bars represent: A–F, H & I, 20 μm; G, 5 μm. 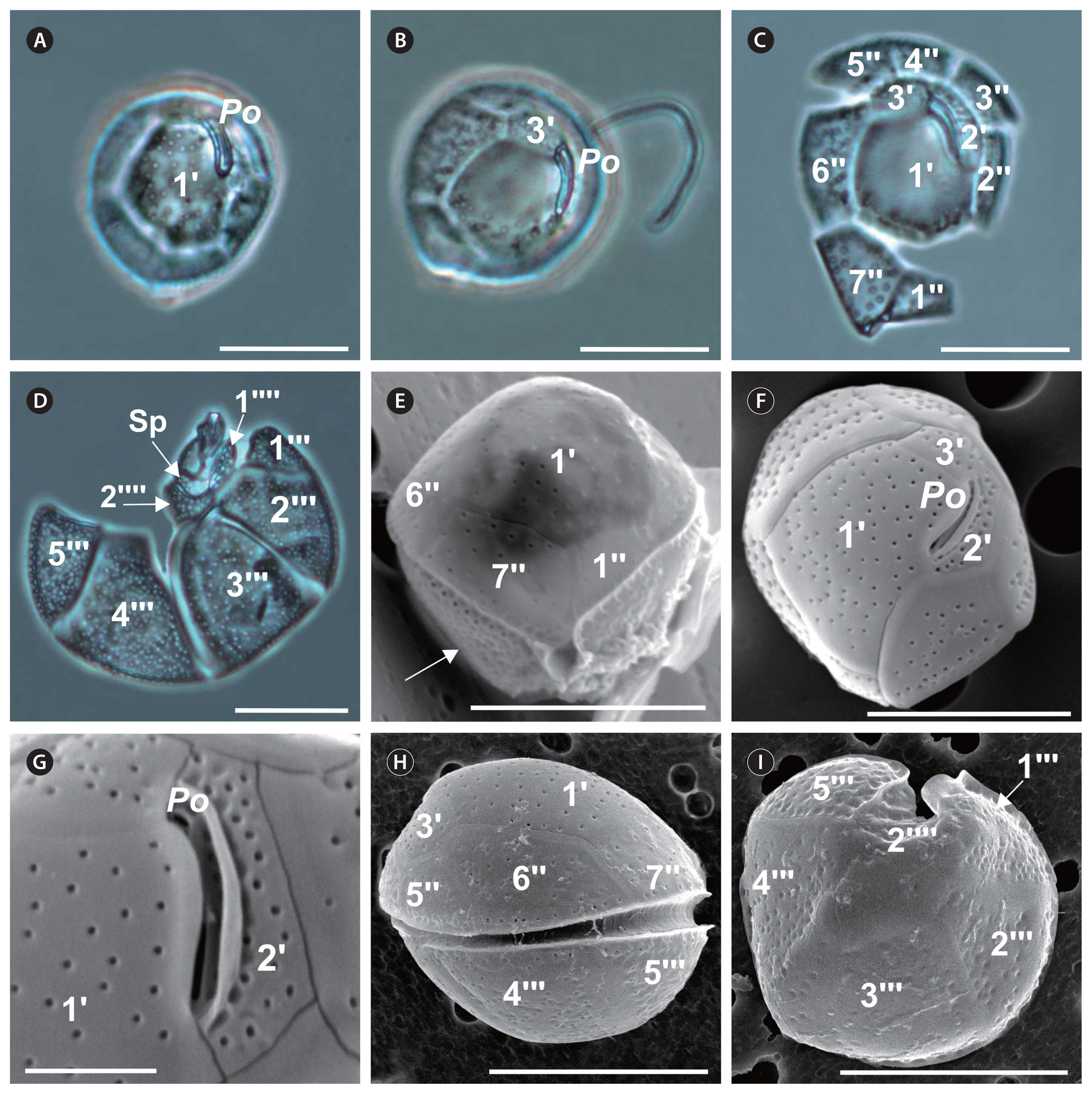
Fig. 6Molecular phylogeny of Coolia using Bayesian inference based on partial large subunit rRNA (D1–D2 region) gene sequences. New sequences are marked in red. Eight species and one lineage were labeled and indicated with vertical lines. The scale bar indicates the number of nucleotide substitutions per site; branch length is shown to scale. Numbers adjacent to each branch indicate the statistical support (left: Bayesian posterior probabilities; right: maximum likelihood bootstrap support values), to avoid overlap some numbers are located outside the branches and their position is indicated by arrows. Bayesian posterior probabilities lower than 0.9 and bootstrap support values lower than 50 are not shown. Dotted line means a half-length. Asterisks indicate maximum likelihood bootstrap support value of 100% and a posterior probability of 1.0. 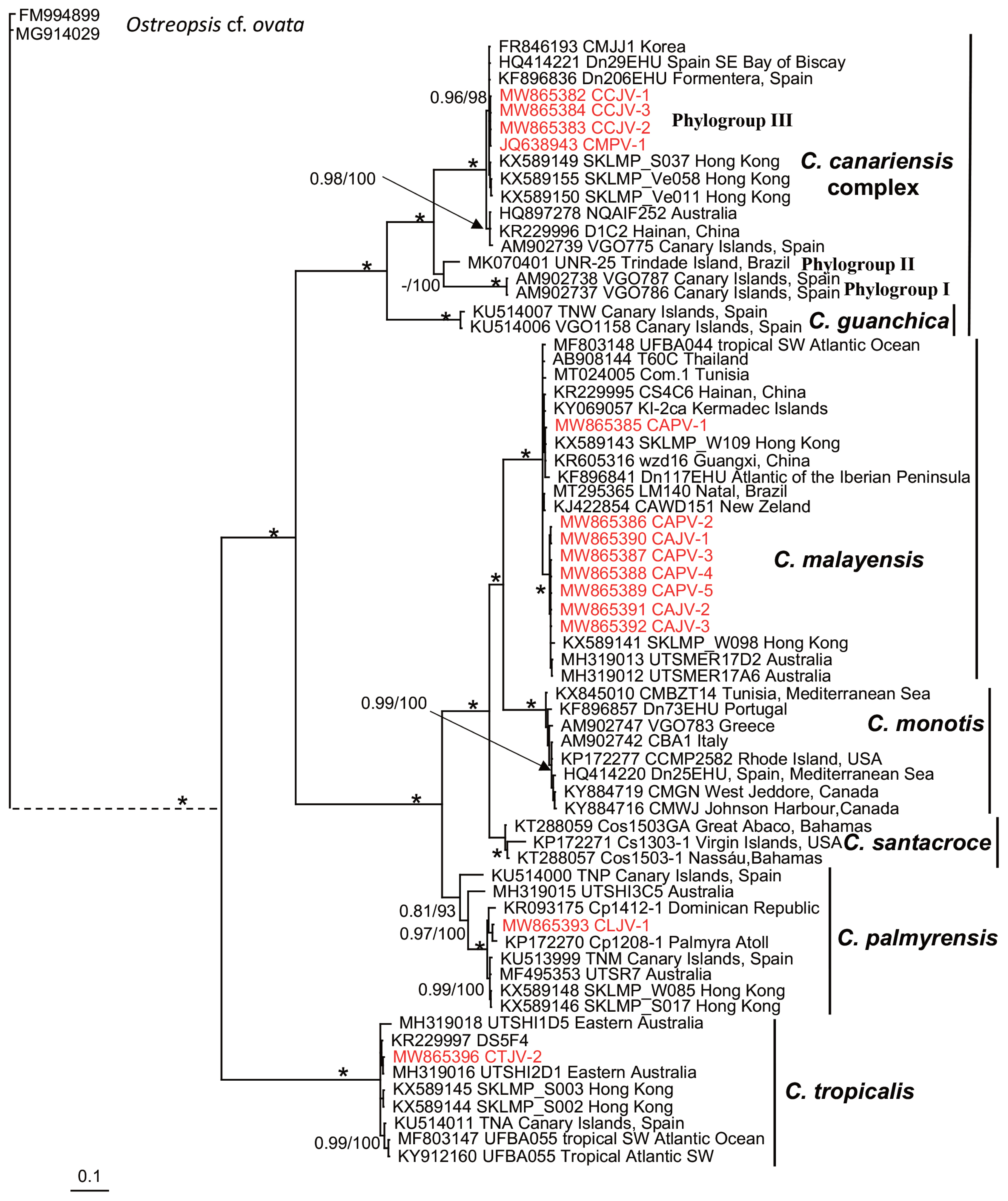
Fig. 7Molecular phylogeny of Coolia using Bayesian inference based on the internal transcribed spacer (ITS) 1–5.8S-ITS2 rDNA gene sequences. New sequences are marked in red. Eight species and one lineage were labeled and indicated with vertical lines. The scale bar indicates the number of nucleotide substitutions per site; branch length is shown to scale. Numbers adjacent to each branch indicate the statistical support (left: Bayesian posterior probabilities; right: maximum likelihood bootstrap support values), to avoid overlap some numbers are located outside the branches and their position is indicated by arrows. Bayesian posterior probabilities lower than 0.9 and bootstrap support values lower than 50 are not shown. Asterisk indicate maximum likelihood bootstrap support value of 100% and a posterior probability of 1.0. 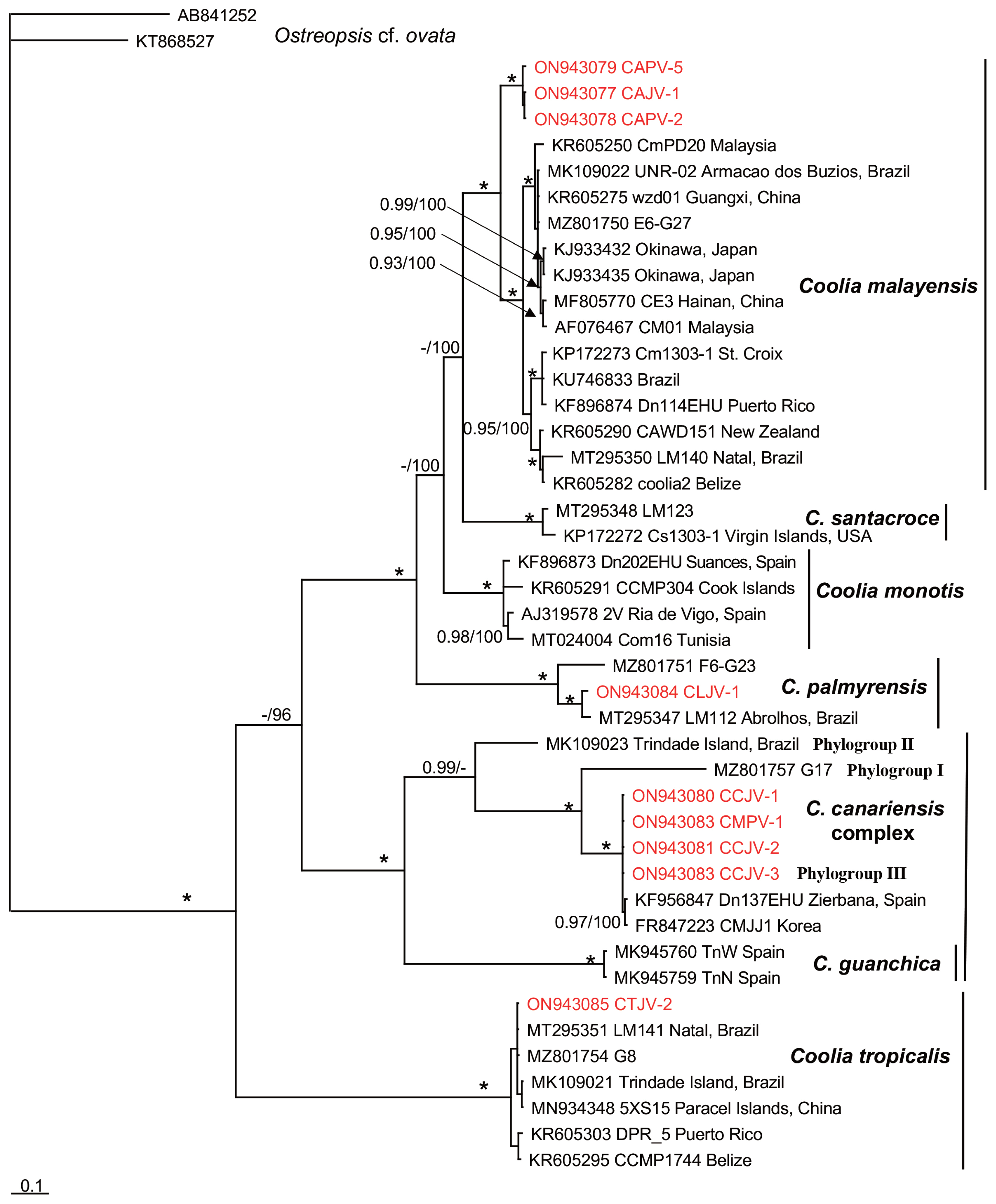
Table 1General information on Coolia strains isolated from Bahía de La Paz, southern Gulf of California Superscript numbers indicate the location of sampling sites on the map (see also Fig. 1). Table 2Morphometric characteristics of Coolia strains isolated from Bahía de La Paz, Gulf of California Table 3GenBank accession numbers assigned to the obtained Coolia sequences Table 4Pairwise genetic distances between Coolia species / strains based on LSU rDNA (D1–D2) sequences Table 5Pairwise genetic distances between Coolia species / strains based on ITS sequences REFERENCESAbdennadher, M., Zouari, AB., Medhioub, W., Penna, A. & Hamza, A. 2021. Characterization of Coolia spp. (Gonyaucales, Dinophyceae) from Southern Tunisia: first record of Coolia malayensis in the Mediterranean Sea. Algae. 36:175–193.
Accoroni, S., Romagnoli, T., Penna, A., Capellacci, S., Ciminiello, P., Dell’Aversano, C., Tartaglione, L., Abboud-Abi Saab, M., Giussani, V., Asnaghi, V., Chiantore, M. & Totti, C. 2016.
Ostreopsis fattorussoi sp. nov. (Dinophyceae), a new benthic toxic Ostreopsis species from the eastern Mediterranean Sea. J Phycol. 52:1064–1084.
Adachi, M., Sako, Y. & Ishida, Y. 1996. Analysis of Alexandrium (Dinophyceae) species using sequences of the 5.8S ribosomal DNA and internal transcribed spacer regions. J Phycol. 32:424–432.
Akselman, R. & Fraga, S. 2022. Other Gonyaulacales, IOC-UNESCO taxonomic reference list of harmful micro algae, Available from: https://www.marinespecies.org/hab
. Accessed Jun 20, 2022
Aligizaki, K. & Nikolaidis, G. 2006. The presence of the potentially toxic genera Ostreopsis and Coolia (Dinophyceae) in the North Aegean Sea, Greece. Harmful Algae. 5:717–730.
Almazán-Becerril, A., Escobar-Morales, S., Rosiles-González, G. & Valadez, F. 2015. Benthic-epiphytic dinoflagellates from the northern portion of the Mesoamerican Reef System. Bot Mar. 58:115–128.
Almazán-Becerril, A., Rosiles-González, G., Escobar-Morales, S., Rodríguez-Palacios, M. & Hernández-Becerril, DU. 2012. Dinoflagelados bentónicos del Arrecife Mesoamericano: Caribe Mexicano. Informe final SNIB-CONABIO. Proyecto No. HJ033, Available from: https://www.gbif.org/es/dataset/ad9da392-a0ae-492f-ad51-24bf0fe1264c(in Spanish). Accessed Jun 20, 2022
Anderson, DM., Kulis, DM. & Binder, BJ. 1984. Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: cyst yield in batch cultures. J Phycol. 20:418–425.
Benson, DA., Cavanaugh, M., Clark, K., Karsch-Mizrachi, I., Lipman, DJ., Ostell, J. & Sayers, EW. 2013. GenBank. Nucleic Acids Res. 41:D36–D42.
Boc, A., Diallo, AB. & Makarenkov, V. 2012. T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 40:W573–W579.
Bomber, JW. & Aikman, KE. 1989. The Ciguatera Dinoflagellates. Biol Oceanogr. 6:291–311.
Caruana, AMN. & Amzil, Z. 2018. Microalgae and toxins. In : Levine IA, Fleurence J, editors Microalgae in Health and Disease Prevention. Elsevier, Amsterdam, 263–305.
David, H., Laza-Martínez, A., Miguel, I. & Orive, E. 2014. Broad distribution of Coolia monotis and restricted distribution of Coolia cf. canariensis (Dinophyceae) on the Atlantic coast of the Iberian Peninsula. Phycologia. 53:342–352.
David, H., Laza-Martínez, A., Rodríguez, F., Fraga, S. & Orive, E. 2020.
Coolia guanchica sp. nov. (Dinophyceae) a new epibenthic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Eur J Phycol. 55:76–88.
de Queiroz-Mendes, M., de Castro-Nunes, J., Fraga, S., Rodríguez, F., Franco, JM., Riobó, P., Branco, S. & Menezes, M. 2019. Morphology, molecular phylogeny and toxinology of Coolia and Prorocentrum strains isolated from the tropical South Western Atlantic Ocean. Bot Mar. 62:125–140.
Doblin, MA., Blackburn, SI. & Hallegraeff, GM. 1999. Comparative study of selenium requirements of three phytoplankton species: Gymnodinium catenatum, Alexandrium minutum (Dinophyta) and Chaetoceros cf. tenuissimus (Bacillariophyta). J Plankton Res. 21:1153–1169.
Dolapsakis, NP., Kilpatrick, MW., Economou-Amilli, A. & Tafas, T. 2006. Morphology and rDNA phylogeny of a Mediterranean Coolia monotis (Dinophyceae) strain from Greece. Sci Mar. 70:67–76.
Faust, MA. 1995. Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. J Phycol. 31:996–1003.
Fraga, S., Penna, A., Bianconi, I., Paz, B. & Zapata, M. 2008.
Coolia canariensis sp. nov. (Dinophyceae), a new nontoxic epiphytic benthic dinoflagellate from the Canary Islands. J Phycol. 44:1060–1070.
Fraga, S. & Rodríguez, F. 2014. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with description of Gambierdiscus silvae sp. nov., a new potentially toxic epiphytic benthic dinoflagellate. Protist. 165:839–853.
Fraga, S., Rodríguez, F., Bravo, I., Zapata, M. & Marañón, E. 2012. Review of the main ecological features affecting benthic dinoflagellate blooms. Cryptogam Algol. 33:171–179.
Fraga, S., Rodríguez, F., Caillaud, A., Diogène, J., Raho, N. & Zapata, M. 2011.
Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae. 11:10–22.
Fraga, S., Rodríguez, F., Riobó, P. & Bravo, I. 2016.
Gambierdiscus balechii sp. nov. (Dinophyceae), a new benthic toxic dinoflagellate from the Celebes Sea (SW Pacific Ocean). Harmful Algae. 58:93–105.
Gárate-Lizárraga, I., González-Armas, R., Verdugo-Díaz, G., Okolodkov, YB., Pérez-Cruz, B. & Díaz-Ortíz, JA. 2019. Seasonality of the dinoflagellate Amphidinium cf. carterae (Dinophyceae: Amphidiniales) in Bahía de La Paz, Gulf of California. Mar Pollut Bull. 146:532–541.
GEOHAB 2012. Global ecology and oceanography of harmful algal blooms. GEOHAB Core Research Project. In : Berdalet E, Tester P, Zingone A, editors Harmful Algal Blooms in Benthic Systems. IOC of UNESCO and SCOR, Paris and Newark, 1–64.
Gómez, F., Qiu, D., Otero-Morales, E., Lopes, RM. & Lin, S. 2016. Circumtropical distribution of the epiphytic dinoflagellate Coolia malayensis (Dinophyceae): morphology and molecular phylogeny from Puerto Rico and Brazil. Phycol Res. 64:194–199.
Guillard, RRL. & Hargraves, PE. 1993.
Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia. 32:234–236.
Guillard, RRL. & Ryther, JH. 1962. Studies on marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervaceae (Cleve) Gran. Can J Microbiol. 8:229–239.
Guiry, MD. & Guiry, GM. 2021. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway, Available from: http://www.algaebase.org
. Accessed Dec 10, 2021
Hall, TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 41:95–98.
Herrera-Sepúlveda, A., Hernández-Saavedra, NY., Medlin, LK. & West, N. 2013. Capillary electrophoresis finger print technique (CE-SSCP): an alternative tool for the monitoring activities of HAB species in Baja California Sur Costal. Environ Sci Pollut Res Int. 20:6863–6871.
Herzi, F., Jean, N., Zhao, H., Mounier, S., Mabrouk, HH. & Hlaili, AS. 2013. Copper and cadmium effects on growth and extracellular exudation of the marine toxic dinoflagellate Alexandrium catenella: 3D-fluorescence spectroscopy approach. Chemosphere. 93:1230–1239.
Holmes, MJ., Lewis, RJ., Jones, A. & Hoy, AW. 1995. Cooliatoxin, the first toxin from Coolia monotis (Dinophyceae). Nat. Toxins. 3:355–362.
Hoppenrath, M. 2022. Prorocentrales. IOC-UNESCO taxonomic reference list of harmful micro algae, Available from: http://www.marinespecies.org/hab
. Accessed Jun 17, 2022
Hoppenrath, M., Murray, SA., Chomérat, N. & Horiguchi, T. 2014. Marine benthic dinoflagellates: unveiling their worldwide biodiversity. Kleine Senckenberg-Reihe, Band 54. Schweizerbart Science Publishers, Stuttgart, 276 pp.
Irola-Sansores, ED., Delgado-Pech, B., García-Mendoza, E., Núñez-Vázquez, EJ., Olivos-Ortiz, A. & Almazán-Becerril, A. 2018. Population dynamics of benthic-epiphytic dinoflagellates on two macroalgae from coral reef systems of the Northern Mexican Caribbean. Front Mar Sci. 5:487 pp.
Jang, SH., Jeong, HJ. & Yoo, YD. 2018.
Gambierdiscus jejuensis sp. nov., an epiphytic dinoflagellate from the waters of Jeju Island, Korea, effect of temperature on the growth, and its global distribution. Harmful Algae. 80:149–157.
Jeong, HJ., Yih, W., Kang, NS., Lee, SY., Yoon, EY., Yoo, YD., Kim, HS. & Kim, JH. 2012. First report of the epiphytic benthic dinoflagellates Coolia canariensis and Coolia malayensis in the waters off Jeju Island, Korea: morphology and rDNA sequences. J Eukaryot Microbiol. 59:114–133.
Karafas, S., York, R. & Tomas, C. 2015. Morphological and genetic analysis of the Coolia monotis species complex with the introduction of two new species, Coolia santacroce sp. nov. and Coolia palmyrensis sp. nov. (Dinophyceae). Harmful Algae. 46:18–33.
Katoh, K. & Standley, DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
Kofoid, CA. 1909. On Peridinium steini Jorgensen, with a note on the nomenclature of the skeleton of the Peridinidae. Arch Protistenk. 16:25–47.
Kretzschmar, AL., Verma, A., Harwood, T., Hoppenrath, M. & Murray, S. 2017. Characterization of Gambierdiscus lapillus sp. nov. (Gonyaulacales, Dinophyceae): a new toxic dinoflagellate from the Great Barrier Reef (Australia). J Phycol. 53:283–297.
Larsson, ME., Smith, KF. & Doblin, MA. 2019. First description of the environmental niche of the epibenthic dinoflagellate species Coolia palmyrensis, C. malayensis, and C. tropicalis (Dinophyceae) from Eastern Australia. J Phycol. 55:565–577.
Laza-Martínez, A., Orive, E. & Miguel, I. 2011. Morphological and genetic characterization of benthic dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum from the south-eastern Bay of Biscay. Eur J Phycol. 46:45–65.
Leaw, C-P., Lim, P-T., Cheng, K-W., Ng, B-K. & Usup, G. 2010. Morphology and molecular characterization of a new species of thecate benthic dinoflagellate, Coolia malayensis sp. nov. (Dinophyceae). J Phycol. 46:162–171.
Leaw, CP., Tan, TH., Lim, HC., Teng, ST., Yong, HL., Smith, KF., Rhodes, L., Wolf, M., Holland, WC., Vandersea, MW., Litaker, RW., Tester, PA., Gu, H., Usup, G. & Lim, PT. 2016. New scenario for speciation in the benthic dinoflagellate genus Coolia (Dinophyceae). Harmful Algae. 55:137–149.
Leung, PTY., Yan, M., Yiu, SKF., Lam, VTT., Ip, JCH., Au, MWY., Chen, C-Y., Wai, T-C. & Lam, PKS. 2017. Molecular phylogeny and toxicity of harmful benthic dinoflagellates Coolia (Ostreopsidaceae, Dinophyceae) in a sub-tropical marine ecosystem: the first record from Hong Kong. Mar Pollut Bull. 124:878–889.
Lewis, NI., Wolny, JL., Achenbach, JC., Ellis, L., Pitula, JS., Rafuse, C., Rosales, DS. & McCarron, P. 2018. Identification, growth and toxicity assessment of Coolia Meunier (Dinophyceae) from Nova Scotia, Canada. Harmful Algae. 75:45–56.
Li, X., Yan, M., Gu, J., Lam, VTT., Wai, T-C., Baker, DM., Thompson, PD., Yiu, SKF., Lam, PKS. & Leung, PTY. 2020. The effect of temperature on physiology, toxicity and toxin content of the benthic dinoflagellate Coolia malayensis from a seasonal tropical region. Water Res. 185:116264 pp.
Litaker, RW., Vandersea, MW., Faust, MA., Kibler, SR., Chinain, M., Holmes, MJ., Holland, WC. & Tester, PA. 2009. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia. 48:344–390.
Lundholm, N., Churro, C., Fraga, S., Hoppenrath, M., Iwataki, M., Larsen, J., Mertens, K., Moestrup, Ø. & Zingone, A. 2009. onwards. IOC-UNESCO taxonomic reference list of harmful micro algae, Available from: https://www.marinespecies.org/hab
. Accessed Jun 20, 2022
Meunier, A. 1919. Microplancton de la mer Flamande. 3me partie. Les Péridiniens Mém Mus R Hist Nat Belg. 8:1–111.
Mitrovic, SM., Fernández-Amandi, M., McKenzie, L., Furey, A. & James, KJ. 2004. Effects of selenium, iron and cobalt addition to growth and yessotoxin production of the toxic marine dinoflagellate Protoceratium reticulatum in culture. J Exp Mar Biol Ecol. 313:337–351.
Mohammad-Noor, N., Moestrup, Ø., Lundholm, N., Fraga, S., Adam, A., Holmes, MJ. & Saleh, E. 2013. Autecology and phylogeny of Coolia tropicalis and Coolia malayensis (Dinophyceae), with emphasis on taxonomy of C. tropicalis based on light microscopy, scanning electron microscopy and LSU rDNA. J Phycol. 49:536–545.
Momigliano, P., Sparrow, L., Blair, D. & Heimann, K. 2013. The diversity of Coolia spp. (Dinophyceae Ostreopsidaceae) in the Central Great Barrier Reef region. PLoS ONE. 8:e79278 pp.
Morquecho, L. & Reyes-Salinas, A. 2004. Colección de Dinoflagelados Marinos (CODIMAR). Centro de Investigaciones Biológicas del Noroeste, S.C. La Paz, Baja California Sur, México, Available from: https://www.cibnor.gob.mx/investigacion/colecciones-biologicas/codimar
. Accessed Jun 20, 2022
Murray, JS., Nishimura, T., Finch, SC., Rhodes, LL., Puddick, J., Harwood, DT., Larsson, ME., Doblin, MA., Leung, P., Yan, M., Rise, F., Wilkins, AL. & Prinsep, MR. 2020. The role of 44-methylgambierone in ciguatera fish poisoning: acute toxicity, production by marine microalgae and its potential as a biomarker for Gambierdiscus spp. Harmful Algae. 97:101853 pp.
Nascimento, SM., da Silva, RAF., Oliveira, F., Fraga, S. & Salgueiro, F. 2019. Morphology and molecular phylogeny of Coolia tropicalis, Coolia malayensis and a new lineage of the Coolia canariensis species complex (Dinophyceae) isolated from Brazil. Eur J Phycol. 54:484–496.
Nishimura, T., Sato, S., Tawong, W., Sakanari, H., Yamaguchi, H. & Adachi, M. 2014. Morphology of Gambierdiscus scabrosus sp. nov. (Gonyaulacales): a new epiphytic toxic dinoflagellate from coastal areas of Japan. J Phycol. 50:506–514.
Núñez-Vázquez, EJ., Almazán-Becerril, A., López-Cortés, DJ., Heredia-Tapia, A., Hernández-Sandoval, FE., Band-Schmidt, CJ., Bustillos-Guzmán, JJ., Gárate-Lizárraga, I., García-Mendoza, E., Salinas-Zavala, CA. & Cordero-Tapia, A. 2019. Ciguatera in Mexico (1984–2013). Mar Drugs. 17:13 pp.
Núñez-Vázquez, EJ., Heredia-Tapia, A., Pérez-Urbiola, JC., Alonso-Rodríguez, R., Arellano-Blanco, J., Cordero-Tapia, A., Pérez-Linares, J. & Ochoa, JL. 2013. Evaluation of dinoflagellate toxicity implicated in recent HAB events in the Gulf of California, Mexico. In : In : Holland P, Rhodes L, Brown L, editors In: Proceedings from HABTech 2003, APEC. A workshop on technologies for monitoring of harmful algal blooms and marine biotoxins. Report no 906; Cawthron Institute Nelson, 94 pp.
Okolodkov, YB., Campos-Bautista, G., Gárate-Lizárraga, I., González-González, JAG., Hoppenrath, M. & Arenas, V. 2007. Seasonal changes of benthic and epiphytic dinoflagellates in the Veracruz reef zone, Gulf of Mexico. Aquat Microb Ecol. 47:223–237.
Okolodkov, YB. & Gárate-Lizárraga, I. 2006. An annotated checklist of dinoflagellates (Dinophyceae) from the Mexican Pacific. Acta Bot Mex. 74:1–154.
Penna, A., Vila, M., Fraga, S., Giacobbe, MG., Andreoni, F., Riobó, P. & Vernesi, C. 2005. Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the western Mediterranean Sea based on morphology, toxicity and internal transcribed spacer 5.8S rDNA sequences. J Phycol. 41:212–225.
Piñón-Gimate, A., Chávez-Sánchez, T., Mazariegos-Villarreal, A., Balart, EF. & Serviere-Zaragoza, E. 2020. Species richness and composition of macroalgal assemblages of a disturbed coral reef in the Gulf of California, Mexico. Acta Bot Mex. 127:e1653 pp.
Ramos-Santiago, AE. 2021. Taxonomía integrativa y crecimiento de Coolia Meunier (Dinophyceae) en extractos de Dictyota dichotoma (Hudson) J.V. Lamouroux (Phaeophyceae) de la Bahía de La Paz, B.C.S. México. B.S. thesis. Universidad del Mar, Campus Puerto Ángel, Oaxaca, Mexico, 91(in Spanish).
Reguera, B., Alonso, R., Moreira, A. & Méndez, S. 2011. Guía para el diseño y puesta en marcha de un plan de seguimiento de microalgas productoras de toxinas. Manuales y Guías de la COI, 59. COI de UNESCO and OIEA, Paris and Viena, 46(in Spanish).
Rhodes, L. & Smith, KF. 2018. A checklist of the benthic and epiphytic marine dinoflagellates of New Zealand, including Rangitāhua/Kermadec Islands. N Z J Mar Freshw Res. 53:258–277.
Rhodes, L., Smith, KF., Verma, A., Curley, BG., Harwood, DT., Murray, S., Kohli, GS., Solomona, D., Rongo, T., Munday, R. & Murray, SA. 2017. A new species of Gambierdiscus (Dinophyceae) from the south-west Pacific: Gambierdiscus honu sp. nov. Harmful Algae. 65:61–70.
Ronquist, F. & Huelsenbeck, JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574.
Scholin, CA., Herzog, M., Sogin, M. & Anderson, DM. 1994. Identification of group and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J Phycol. 30:999–1011.
Sepúlveda-Villarraga, M. 2017. Dinoflagelados potencialmente tóxicos asociados a macroalgas en la Bahía de La Paz, B.C.S. M.S. thesis. Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas (CICIMAR-IPN), La Paz, Mexico, 69(in Spanish).
Smith, KF., Rhodes, L., Verma, A., Curley, BG., Harwood, DT., Kohli, GS., Solomona, D., Rongo, T., Munday, R. & Murray, SA. 2016. A new Gambierdiscus species (Dinophyceae) from Rarotonga, Cook Islands: Gambierdiscus cheloniae sp. nov. Harmful Algae. 60:45–56.
Stamatakis, A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.
Swofford, D. 2002. 2002PAUP* Phylogenetic analysis using parsimony (* and other methods), version 4 b10. Sinauer Associates, Sunderland MA.
Ten-Hage, L., Turquet, J., Quod, JP. & Couté, A. 2000.
Coolia areolata sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the southwestern Indian Ocean. Phycologia. 39:377–383.
Tester, PA., Litaker, RW. & Berdalet, E. 2020. Climate change and harmful benthic microalgae. Harmful Algae. 91:101655 pp.
Tibiriçá, CEJA., Sibat, M., Fernandes, LF., Bilien, G., Chomérat, N., Hess, P. & Mafra, LL. Jr 2020. Diversity and toxicity of the genus Coolia Meunier in Brazil, and detection of 44-methyl gambierone in Coolia tropicalis
. Toxins. 12:327 pp.
Vargas-Montero, M., Morales, A. & Cortés, J. 2012. First report of the genus Gambierdiscus (Dinophyceae) and other benthic dinoflagellates from Isla del Coco National Park, Costa Rica, Eastern Tropical Pacific. Rev Biol Trop. 60:187–199, (in Spanish).
|
|
|||||||||||||||||||||||||||||||||||||||