Abbreviations
CDPK
calcium calmodulin-dependent protein kinase
DAMP
damage-associated molecular pattern
ETI
effector-triggered immunity
HR
hypersensitive response
LRR
leucine-rich repeat
PAMP
pathogen-associated molecular pattern
PRR
pattern recognition receptor
PTI
pattern-triggered immunity
ROS
reactive oxygen species
TIR
Toll/interleukin-1 receptor
INTRODUCTION
Algae are the most important primary producers, sequestering more than half of the world’s net carbon and producing about 52 giga tons of organic carbon per year (Hallmann 2007, Ullmann and Grimm 2021). Among these, seaweeds, multicellular algae, are cultivated in many parts of the world, and in 2019 alone, 34.7 million tons of seaweed were produced, valued at approximately US$14.7 billion, and used to produce food, food additives (e.g., hydrocolloids), animal feed, nutritional supplement, cosmetics, and pigments (Borowitzka 2013, Cai et al. 2021, Ullmann and Grimm 2021). However, the sustainability of the seaweed aquaculture industry faces a number of challenges, including rising water temperatures and more frequent strong weather events, which can lead to damage to facilities and disease outbreaks (Cottier-Cook et al. 2021).
Disease outbreaks in aquaculture farms can be caused by pathogens introduced from outside the farms and conversely the farms can act as pathogen pools from which pathogens can spread to the surrounding environment (Tedesco et al. 2021, Roh and Kannimuthu 2023). In recent years, there has been a growing concern about the potential threat of pathogens spreading from aquaculture farms and affecting coastal seaweed populations (Bouwmeester et al. 2021), as well as the adverse effects that treatments used by aquaculture farms to control pathogens may have on the surrounding environment (Kim et al. 2014). Currently, a significant number of commercially important seaweeds belong to the red algae, and despite their much longer history of aquaculture compared to other algae, little is known about how pathogens interact with red algae to cause disease and which genes are involved in the red algal defense against pathogens (Im et al. 2019). As a result, the methods currently used to control disease in seaweed farms are not the result of systematic basic scientific research, but have been developed based on farmers’ experience, and may have little effect and may even pose a risk to the surrounding environment (Kamoun et al. 2015).
In order to develop effective disease control measures, we need to better understand the cellular and molecular mechanisms of host-pathogen interactions in red algae and the scientific basis of currently used treatments (Fawke et al. 2015). In this article, we list the known pathogens that infect red algae, with a focus on oomycetes, and review recent research on the molecular mechanisms of host-pathogen interactions and disease control methods currently used in aquaculture.
OOMYCETE PATHOGENS THAT INFECT RED ALGAE
Oomycetes are a diverse group of fungus-like eukaryotic microorganisms, also known as water molds (Beakes et al. 2012). Because of their filamentous growth habit, nutrient supply by absorption, flagellate stages, and reproduction by spores, oomycetes were long considered “lower” fungi (Latijnhouwers et al. 2003). However, it is now clear that this group of organisms is not related to fungi but is more closely related to diatoms and brown algae in the clade Stramenopiles (Beakes and Thines 2017, Adl et al. 2019).
Oomycetes are one of the most important aquatic pathogens, devastating important agricultural crops and natural ecosystems (Hardham 2007, Kamoun et al. 2015, Badis et al. 2019, 2020). Oomycetes are capable of infecting hosts ranging from algae, plants, and animals (Gachon et al. 2010, Saraiva et al. 2023). Several pathogenic oomycetes are responsible for massive destruction and losses in agriculture and aquaculture (Phillips et al. 2008, Kim et al. 2014). However, taxonomic studies of marine oomycetes began much later and are less well informed than those of other oomycetes. It was not until 1960s that Sparrow cataloged the oomycetes infecting red algae and classified the species based on morphology and life history (Sparrow 1960). He recorded a total of seven species infecting red algae: Eurychasmidium tumefaciens, E. sacculum (formerly known as Eurychasma sacculus), Sirolpidium andreei (formerly known as Olpidiopsis andreei), Petersenia lobata, P. pollagaster, Pontisma lagenidioides, and Pythium marinum. Only two of these species, Pyt. marinum and Po. lagenidioides, have been taxonomically studied using molecular methods (LeVesque and De Cock 2004, Hyde et al. 2014, Buaya et al. 2019).
Molecular data on Pyt. marinum were first reported by LeVesque and De Cock (2004). Pyt. marinum is morphologically distinguished from other Pythium species by a single linear antheridium that tapers abruptly (Sparrow 1934, 1960). The internal transcribed spacers (1 and 2) and the 5.8S gene of Pyt. marinum (strain number: CBS 750.96) were identical to Pyt. coloratum, Pyt. lutarium, and Pyt. dissotocum but differed by 1 bp from Pyt. diclinum (LeVesque and De Cock 2004). In further studies, the large and small subunits of nrRNA, the cytochrome c oxidase subunit 2 gene (cox2), and β-tubulin were also used in the same strain of P. marinum (Hyde et al. 2014). Pyt. marinum showed a little difference from other Pythium species in a phylogenetic analysis based on these sequences (Hyde et al. 2014). Questions have been raised as to whether this strain is the Pyt. marinum reported by Sparrow (1934), as the strain used in the molecular phylogenetic study was isolated from soil rather than a marine environment where the species was originally reported (Hyde et al. 2014), but where the strain was isolated may not be a major issue as there are reports that Pyt. porphyrae, a member of the same genus, can survive in both freshwater and seawater and can infect both terrestrial plants and marine red algae (Klochkova et al. 2017a). Recently, a Pythium sp. infecting the green algae Ulva spp. and terrestrial grass species in low salinity environments, was discovered in the natural environment in Norway, supporting the above ideas that terrestrial oomycete pathogens can be introduced into intertidal algal communities or vice versa (Herrero et al. 2020).
After Sparrow (1960), fourteen new species or varieties of oomycetes infecting red algae were discovered. Two species were classified as new only morphologically (i.e., Olpidiopsis antithamnionis, P. palmariae) (Whittick and South 1972, Van der Meer and Pueschel 1985), and nine species were morphologically and molecularly identified (i.e., Olpidiopsis porphyrae, Sekimoto et al. 2008; O. feldmanni, Aleem 1952, Fletcher et al. 2015; O. bostrychiae, Sekimoto et al. 2009; O. pyropiae, Klochkova et al. 2016; O. heterosiphoniae, Klochkova et al. 2017b; O. palmariae, Badis et al. 2019; O. muelleri, Badis et al. 2019; Pythium chondricola, Lee et al. 2015, 2017, Lee and Lee 2022; and Pyt. porphyrae, Takahashi et al. 1977, Diehl et al. 2017, Qiu et al. 2019). Three varieties were also identified (O. muelleri var. polysiphoniae, Badis et al. 2019; O. porphyrae var. scotiae, Badis et al. 2019; and O. porphyrae var. koreanae, Kwak et al. 2017). These varieties are not distinguished by the 18S rRNA sequence but are distinguished from other varieties morphologically and in their host infection characteristics. For example, O. muelleri var. polysiphoniae forms a sporangium cell wall with a honeycomb structure that is different from that of O. muelleri (Badis et al. 2019). The 18S rRNA sequences of O. porphyrae var. porphyrae and O. porphyrae var. koreanae were identical but differs in their conserved introns, while O. porphyrae var. scotiae differs in both conserved introns and 18S rRNA sequences (Badis et al. 2019).
Accumulating molecular phylogenetic information has led to a need to reclassify marine oomycetes, particularly the genus Olpidiopsis. An oomycete believed to be Po. lagenidioides was collected from the red algae Ceramium rubrum in Drøbak, Oslo Fjord, Norway in 2017 (Buaya et al. 2019) and was originally recorded as infecting Ceramium spp. (Sparrow 1960). Morphologically similar to Po. lagenidioides recorded by Sparrow (1960), 18S rDNA sequences were analyzed to construct molecular phylogenies (Buaya et al. 2019). The phylogeny results showed Po. lagenidioides was within rhodophyte-infecting members of Olpidiopsis, forming a clade but without support (Buaya et al. 2019). Within the revised genus Pontisma, only Po. lagenidioides is known to form large hyphal thalli, but the thallus segments in that species develop in a very similar manner as compared to the species in which the thallus remains smaller (Buaya et al. 2019, 2021). Olpidiopsis saprolegeniae, infecting another oomycete, was distantly related to the red algal pathogens (Buaya et al. 2019), and the genus Pontisima was proposed for these pathogens (Buaya et al. 2019, Buaya and Thines 2020). Po. lagenidioides grouped together with other the red algal pathogens (i.e., O. feldmanni, O. heterosiphoniae, O. pyropiae, O. porphyrae, and O. porphyrae var. koreanae), but this relationship was not supported by 18S rDNA sequences (Buaya et al. 2019, 2021). More work is needed before any taxonomic changes are made.
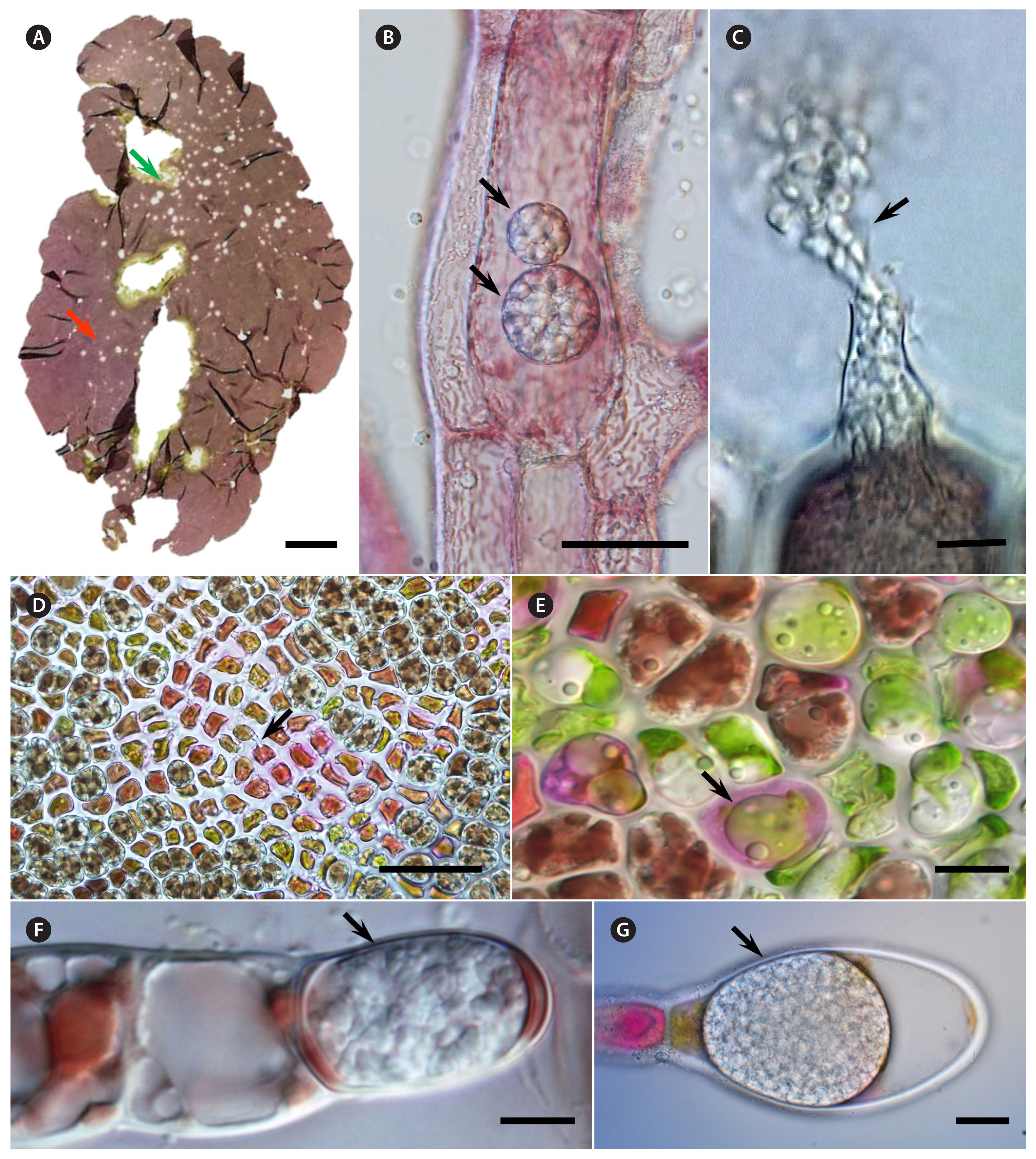
In some cases, oomycetes that have been considered distinct species are not well supported by molecular phylogenetic evidence. For example, Pyt. porphyrae is the first oomycete pathogen identified in seaweed farms and is the causative agent of red rot disease, one of the most common and severe diseases in Pyropia farms in China, Korea, and Japan (Arasaki 1947, Takahashi et al. 1977, Park et al. 2001, Ding and Ma 2005) (Fig. 1A & D). The morphology of Pyt. porphyrae and Pyt. chondricola is very similar and there is controversy about their separation into distinct species (Park et al. 2000, Diehl et al. 2017). Pyt. porphyrae and Pyt. chondricola both have filamentous, non-inflated sporangia, similar sexual structures, and both infect Pyropia (LeVesque and De Cock 2004, Lee et al. 2015). The main differences are the considerably lower cardinal temperatures for growth, larger oogonia, and aplerotic oospores in Pyt. chondricola (LeVesque and De Cock 2004). Sequences of the internal transcribed spacer region, currently used in oomycetes taxonomy, are 100% identical (LeVesque and De Cock 2004). The 18s rRNA sequence identified in the recently discovered genome of Pyt. chondricola was 100% identical to that of Pyt. porphyrae (Nguyen et al. 2022). Although there are differences in the cox1 sequence, it is smaller than the regional differences between Pyt. porphyrae from Japan and Korea, so this is ultimately considered insufficient to distinguish between species using cox1 (Lee et al. 2015, 2017, Lee and Lee 2022). In the event of a major outbreak of red rot disease in aquaculture farms, the names of these two species are sometimes used interchangeably, leading to confusion about the need for different treatments for the two pathogens, so it is necessary to clarify their taxonomic status.
Olpidiopsis is a genus of obligate holocarpic endobiotic oomycetes (Buaya et al. 2019), with most species reported to be parasitic within red algal cells, and several of which cause significant economic damage to seaweed farms in Far Eastern countries (Kim et al. 2014). To date, seven non-oosporic Olpidiopsis species have been described as parasites of marine red algae both morphologically and phylogenetically (Table 1). The morphological taxonomy of Olpidiopsis species is based upon a few morphological features such as shape and size of the thallus, the number of discharge tubes, plus host specificity (Klochkova et al. 2012). O. porphyrae var. porphyrae (Sekimoto et al. 2008) and O. porphyrae var. koreanae (Fig. 1A) (Kwak et al. 2017) infects species of the genus Pyropia and Bangia, developing spherical-shaped holocarpic thalli within the cytoplasm of its host (Fig. 1E), and produces monoplanetic, subapically-inserted biflagellate zoospores. The released spores immediately infect neighboring cells, and the disease spreads very quickly (Kim et al. 2014, Klochkova et al. 2016, Kwak et al. 2017), and both varieties are often found together on the same Pyropia blade (Fig. 1A & E). O. porphyrae var. scotiae reported from Scotland infects species of Porphyra (Badis et al. 2019). Olpidiopsis species also infect Ceramialean algae such as Bostrychia moritziana (Fig. 1C), Heterosiphonia pulchra, and Dasysiphonia japonica (Fig. 1B). Species of Olpidiopsis, which develop zoosporangia inside the cells of their hosts, are usual highly host-specific, but can also have multiple hosts, differing only in the rate at which the infection spreads (Klochkova et al. 2012). Olpidiopsis bostrychiae can infect a variety of red algal genera (i.e., Bostrychia spp., Dasysiphonia japonica, Pyropia spp., and Porphyra sp.) and grows best on the genus from which was isolated, Bostrychia (Sekimoto et al. 2009). Olpidiopsis heterosiphoniae can also infect a variety of red algal genera (i.e., Heterosiphonia spp., Dasya sp., Dasysiphonia chejuensis, and Pyropia tenera), but can coexist for long periods of time without completely consuming the host (Klochkova et al. 2017b).
RED ALGAL DEFENSE AGAINST OOMYCETE PATHOGEN
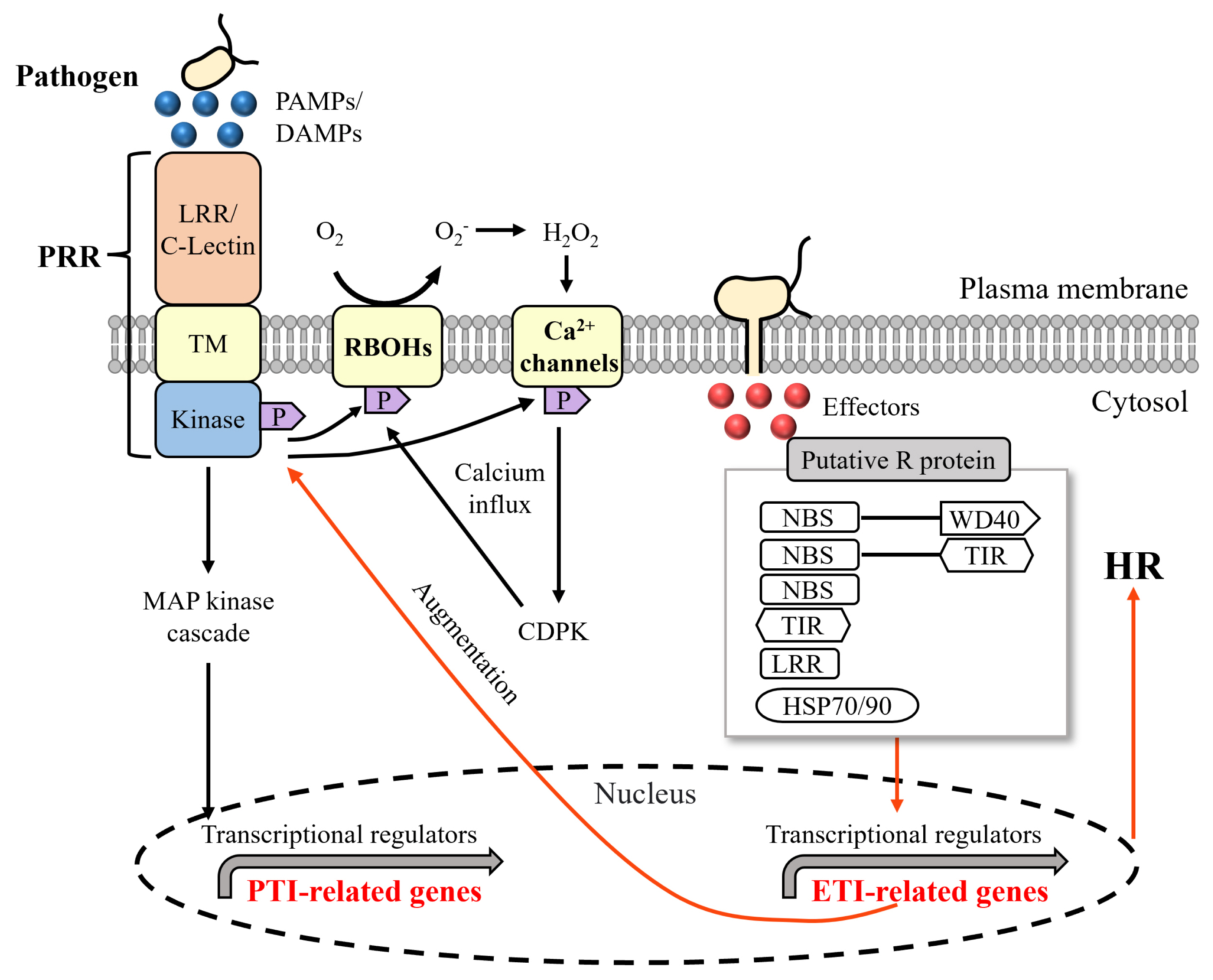
In response to pathogen infection, hosts develop diverse immune responses. An immune response are the molecular steps that organisms use to defend themselves against foreign invaders (Coll et al. 2011). Disease develops when the pathogen is not able to evade these host defenses. Plants and animals, share striking similarities in their innate immune systems, some of which are likely due to convergent evolution (Jones and Dangl 2006, Mayor et al. 2007). Immune responses discriminate self from non-self and activate tightly regulated pre- and post-invasion defense responses to minimize the damage caused by harmful agents (Chakraborty et al. 2017). In both plants and animals, the first line of defense is provided by pattern recognition receptors (PRRs) on the plasma membrane that recognize conserved pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns that activate pattern-triggered immunity (PTI) (Mayor et al. 2007, Shine et al. 2019, Ding et al. 2022). After infection, plants recognize effectors secreted by pathogens via resistance (R) proteins and induce effector-triggered immunity (ETI) (Coll et al. 2011, Chakraborty et al. 2017).
Plants have PRRs that can recognize molecular signatures that identify whole classes of microbes (such as chitin for fungi or peptidoglycan for bacteria) but are absent from the host (Boller and Felix 2009). The best-studied class of plant PRRs are receptor-like kinases, which have an ectodomain of leucine-rich repeats (LRRs). Toll-like receptors are one of the earliest PRRs discovered in animals (Iwasaki and Medzhitov 2010). Since then, many PRRs and their corresponding ligands have been discovered. Recognition of PAMPs by PRRs at the onset of infection activates PTI, which involves Ca2+ signaling, mitogen-activated protein kinase cascades, and reactive oxygen species (ROS) signaling, and can non-specifically control colonization of most pathogens (Ding et al. 2022). However, various pathogens have evolved “effector” proteins that can suppress PTI signaling leading to effector-triggered susceptibility (Bari and Jones 2009). These effector proteins are known as “virulence factors” (Chakraborty et al. 2017).
As genomic and transcriptomic research progresses in red algae, specific pathogen receptor genes homologous to those of other systems have been discovered (Brawley et al. 2017, de Oliveira et al. 2017, Tang et al. 2019). Plant-typical PRR genes (LRR domain containing genes) were identified in Laurencia dendroidea (de Oliveira et al. 2017), Pyropia yezoensis (Tang et al. 2019), and P. tenera (Im et al. 2019). Animal typical PRR genes (C-type lectin domains containing genes) have been found in Porphyra umbilicalis (Brawley et al. 2017), P. yezoensis (Tang et al. 2019), and P. tenera (Im et al. 2019). However, typical plant R proteins with a conserved protein structure of TNL (Toll/interleukin-1 receptor [TIR]-NBS-LRR or nTNL [NBS-LRR]) was not identified in red algae (Ortiz and Dodds 2018). R domain containing proteins were also identified in Porphyra umbilicalis (Brawley et al. 2017), P. yezoensis (Tang et al. 2019), and P. tenera (Im et al. 2019). It has been suggested that HSP90 proteins in P. yezoensis may play a role similar to R proteins in plants (van Bentem et al. 2005, Tang et al. 2019). The defense mechanisms of red algae against oomycete pathogens identified to date are summarised in Fig. 2.
Pathogen receptor genes in red algae are expressed differently between species and depending on the type of pathogen. A transcriptomic study of P. yezoensis found that PRR genes (genes containing C-type lectin domains) and five genes encoding a single R proteins domain (LRR, NBS, or TIR) were upregulated after infection by Pyt. porphyrae (Tang et al. 2019). Microarray studies of P. tenera in response to infection by two oomycete pathogens (Pyt. porphyrae, Olpidiopsis pyropiae) and a viral pathogen (PyroV1) show that PRR genes are specifically upregulated in response to oomycete pathogens (Im et al. 2019) and not the virus. For example, in P. tenera, a PRR gene was upregulated by infection with Pyt. porphyrae, and a gene with an LRR domain was slightly upregulated by infection with P. porphyrae and O. pyropiae. In addition, two genes encoding NBS domain-containing proteins were upregulated upon infection with these oomycete pathogens, but none of these genes was upregulated upon infection with PyroV1. A gene encoding a TIR domain-containing protein was upregulated only upon infection with PyroV1 (Im et al. 2019).
A set of pathogen receptor genes, as found in land plants, have not yet been reported in red algae, but recent studies have reported a number of genes involved in defense responses, including PTI and ETI. In Laurencia dendroidea, genes involved in PTI, such as mitogen-activated protein kinase kinase, calcium calmodulin-dependent protein kinase, NADPH oxidase, and antioxidant enzyme genes, were upregulated in a time-dependent manner after inoculation with the marine bacterium Vibrio madracius (de Oliveira et al. 2017). In P. tenera, several defense-related genes typically considered PTIs in plants, such as heat shock proteins, cell wall-associated hydrolase, and NADPH oxidase, were induced in response to pathogen infection (Im et al. 2019). Similar defense mechanisms have been reported in P. yezoensis, including protective enzymes, heat shock proteins, secondary metabolites, cellulases, protease inhibitors, NADPH-oxidases, and antioxidant enzymes, and these genes were upregulated from the onset of infection by Pyt. porphyrae (Tang et al. 2019). It has also been reported that the hypersensitive response (HR)-related genes such as metacaspase, endonuclease G, and cytochrome C, which are upregulated during ETI, are also induced by infection with oomycete pathogens (Tang et al. 2019).
Calcium-mediated ROS signaling mediates the fertilization and wound-healing responses of red algae and also plays an important role in response to pathogens (Weinberger et al. 2005, Moon et al. 2022, Shim et al. 2022). NADPH oxidase in the cell membrane (called respiratory burst oxidase homolog or RBOH) reduces oxygen to superoxide, which rapidly forms hydrogen peroxide and diffuses from cell to cell through the extracellular space (Torres et al. 2006, Perez and Brown 2014, Peláez-Vico et al. 2022). ROS along with NO (nitric oxide) induces HR-mediated cell death in plants (Swarupa et al. 2014). ROS produced by pathogen-exposed cells trigger ROS / calcium-activated calcium channels in neighboring cells, resulting in auto-propagation throughout the plant, activating systemic acquired adaptation with increased HR reaction (Choudhury et al. 2017, Ding et al. 2022). Accumulation of ROS was also observed in red algae in response to pathogen infection. When the red alga Gracilaria conferta was exposed to oligosaccharides produced by microbial degradation of the agar cell wall, an oxidative burst was observed in the cell membrane as the first physiological sign of a PAMP (Weinberger 1999, Weinberger et al. 2005). ROS accumulation was also observed in P. tenera in response to oomycete as well as virus infection (Im et al. 2019). However, more research is needed to understand when and how ROS signaling is involved in red algal responses to infection by oomycete pathogens.
CURRENT TREATMENT MEASURES FOR OOMYCETE DISEASES
Control methods for oomycete diseases were first developed by seaweed farmers in the field, particularly in Pyropia farming, and developed to industrial levels in Asia after the 1950s. Most of the control methods currently used are based on Pyropia’s high tolerance to abiotic stress (Kim et al. 2014). Sea farmers inhibit the growth of pathogens by exposing aquaculture nets to air for several hours and letting them dry in sunlight. Since the 1970s, a frozen-net method has been developed, in which young blades attached to aquaculture nets are air-dried, frozen at −20°C, and then reintroduced to farms when seawater is around 10°C (e.g., Fujita and Migita 1980, Ding and Ma 2005, and Klochkova et al. 2012). While the above methods are effective in reducing the damage caused by oomycete pathogens to some extent, it is difficult to avoid some reduction in production due to shorter cultivation periods or slower growth (Kim et al. 2023).
Acid washing of cultivation nets is the most commonly used method in Pyropia farms to reduce epiphytic green algae, diatoms, and oomycete pathogens (Park et al. 2001). A boat equipped with a large tub passes underneath the cultivation net and the net is immersed in an acid solution in the tub while the boat slowly moves forward. As Pyropia tolerates acid better than other organisms this treatment has been regarded as useful to clean the cultivation nets, but this method has little effect on Olpidiopsis-blight and is a burden on the environment (Kim et al. 2014). As regulations on aquaculture methods are strengthened, chemical treatments are currently limited to a few organic acids or concentrated salts that have been approved through government review and testing in the field (Kim et al. 2023). Recently, a technology has been developed that can more effectively control oomycete pathogens by washing aquaculture nets with calcium propionate instead of organic acids and is awaiting government review (Kim et al. 2023). Despite all these attempts, oomycete pathogens are still a major issue for Pyropia farms in Far Eastern countries and more research on their control is needed.
CONCLUSIONS AND PERSPECTIVES
Many questions still need to be addressed, or clarified, to improve our understanding of the pathogens of seaweed farms and how their impact can be mitigated. A better understanding of the diversity of pathogens found in the ocean and around seaweed farms is still needed as exploring the environmental DNA in seawater is still uncovering oomycetes related to known red algal pathogens (Badis et al. 2019).
Phylogenetic results show that rhodophyte-infecting members of Olpidiopsis, do not form a supported clade based on standard markers (Buaya et al. 2019). It is very likely that the genus Olpidiopsis currently contains species that are phylogenetically divergent, and more information on host specificity or cell ultrastructure, together with the development of higher-resolution molecular markers, is needed to understand this diversity. Pythium chondricola and Pyt. porphyrae were originally named on the basis of host specificity, but current molecular markers make it very difficult to distinguish between them, leading to the two names being used interchangeably for pathogens found in Pyropia aquaculture in East Asia. Development of higher resolution molecular markers to discriminate between them using strains with host specificity consistent with the original description of the two species is required.
Although aspects of Pyropia spp. innate immunity were shown after infection with oomycetes, research on innate immunity in other red algae is needed. Several economic red algae species have been reported to be infected with oomycetes and warrant further study (Table 1). ROS are important molecules in innate immunity that are also found in red algae (Potin et al. 2002, Weinberger et al. 2005, Im et al. 2019, Tang et al. 2019). Current treatment measures for oomycete diseases mainly involve increasing resistance to abiotic stress. Since abiotic stress is also related to the production of ROS, it is expected that research on ROS signaling will be important in future red algal oomycete diseases.