ABSTRACTSulfated polysaccharides (SPs) isolated from seaweed have emerged as remarkable bioactive compounds with a wide spectrum of biological activities and have substantial value in the scientific and industrial domains. The current study explores the diverse biological activities of SPs and their relationship with their structures. This aids in an in-depth examination of the multifaceted biological activities of SPs, including anticoagulant, anti-inflammatory, antiviral, antioxidant, and immunomodulatory properties, which underpin their potential health benefits. Furthermore, the current study explores the complicated properties of SPs, with their extraction methodologies and techniques for precise characterization. Elucidation of the commercial significance of SPs derived from brown, red, and green seaweed by highlighting their potential applications has emphasized their importance in human well-being. Further, this review emphasizes the challenges needed to overcome research and industrial innovations for SPs. Collaboration among researchers, industry stakeholders, and regulatory authorities can overcome these challenges and elevate the potential of SPs to revolutionize industries such as pharmaceuticals, cosmeceuticals, food, and biotechnology.
INTRODUCTIONSulfated polysaccharides (SPs) isolated from seaweed have gained remarkable attention as a family of natural substances that are widely used in academic and industrial applications. The sulfate groups that are covalently attached to the polysaccharide molecular structure demonstrate several biological activities that distinguish them from other complex polysaccharides, which are present in large amounts in different seaweed species. The research and development of SPs from seaweed has undergone a dynamic evolution owing to continuous scientific understanding, technical breakthroughs, and escalating environmental concerns (Jayawardena et al. 2022, Liyanage et al. 2023b). The significance of SPs in biological systems depends on their diverse roles in physiological processes. Furthermore, their complex structural characteristics enable them to interact with biomolecules and cells in a unique way, contributing to their significance in biological systems (Costa et al. 2010). Furthermore, SPs influence key biological functions and modulate them to play beneficial roles in prominent biological functions such as antioxidation, inflammation, coagulation, viral infection, and wound healing (Costa et al. 2010, Little et al. 2021). Moreover, SPs serve as multifunctional molecules that can facilitate a wide range of biological activities, making them valuable in various industries, such as medicine, pharmaceuticals, cosmeceuticals, and functional food (Hayashi et al. 2008, Jayawardhana et al. 2023, Liyanage et al. 2023a). These beneficial outcomes make seaweed a valuable resource for numerous industrial and scientific research.
Seaweed can be divided into three main categories: red, green, and brown. Each of these seaweed groups contains unique SPs with distinct structures and properties. Furthermore, this diversity allows the extraction of a wide range of SPs with numerous applications (Usov 1992, Ciancia et al. 2010, Costa et al. 2010, Ale et al. 2011, Hwang et al. 2022). Red seaweed (Rhodophyta) produce two sulfated galactans, carrageenan and agaran, which have various applications in the pharmaceutical and food industries. Brown seaweed (Phaeophyta) produce fucoidans, laminarans, and alginates, which have unique sulfate structures and exhibit a wide range of valuable biological activities. Green seaweed (Chlorophyta) contain sulfated ulvans and xylans, which have garnered attention for their potential health benefits. This diversity of SPs derived from seaweed underscores their importance as valuable natural resources with varied applications and motivates ongoing research on their isolation, characterization, and therapeutic potential.
Globally, coastal habitats are rich in seaweed. Furthermore, they thrive in marine environments, and their growth is often rapid and prolific. This natural abundance ensures a consistent and sustainable supply of seaweed for SP extraction (Snethlage et al. 2023). Some of these seaweed species are commercially cultivated. Furthermore, specific seaweed varieties optimized for high polysaccharide yields can be developed by modifying aquaculture techniques (Lüning and Pang 2003). Currently, various methods are available for extracting SPs from different seaweed, including conventional or novel methods and combinations of these two types. Most importantly, these methods ensure the preservation of polysaccharide structural integrity and bioactivity (Ale et al. 2011). Seaweed are considered environmentally friendly. Their cultivation requires limited freshwater, land, and synthetic fertilizers, making them sustainable alternatives to terrestrial crops. Additionally, seaweed can help mitigate ocean acidification and serve as carbon sinks (Wiencke and Bischof 2012). Some seaweed species are edible and are part of the traditional diet in various cultures. In addition to SPs, seaweed are rich in essential vitamins, nutrients, and minerals that contribute to the overall nutrition and health (Wiencke and Bischof 2012). They are a valuable source of SPs because of their diversity, abundance, ease of cultivation, eco-friendliness, and wide range of applications. Their unique properties and sustainable nature make them a compelling resource for both traditional and emerging industries as well as for advancing applied scientific knowledge. This comprehensive review elucidates the multifaceted biological activities and health-related benefits inherent to SPs, while concurrently addressing the methodologies of their extraction and structural characterization. Furthermore, it delves into their diverse industrial applications, associated regulatory considerations, and challenges, and concludes by presenting an insightful outlook on future research prospects within this domain. The content of the manuscript is summarized in Fig. 1.
EXTRACTION AND CHARACTERIZATION OF SULFATED POLYSACCHARIDESExtraction techniques of SPsThe SP extraction procedure is pivotal for obtaining high-quality bioactive compound yields. The selection of the extraction method depends on factors such as the seaweed species, desired polysaccharide type, and intended downstream applications. Several prominent extraction techniques have been developed and employed, each with distinct advantages and limitations (Jayasinghe et al. 2016).
The most conventional method is hot water extraction wherein dried seaweed samples are subjected to high-temperature water treatment. This facilitates the release of SPs into the aqueous phase. This method is simple and cost-effective. However, it entails a prolonged extraction time and necessitates subsequent purification steps to isolate the target SP from other constituents (Shao et al. 2013).
Enzyme-assisted extraction is another widely used method that employs the use of various enzymes, such as cellulase, pectinase, and alginase, to break down the cell wall components of seaweed, which assists in effectively releasing SPs, while preserving their structural integrity. Enzyme-assisted extraction is known for its specificity and yield. However, the need for specific enzymes and longer extraction times may increase their operational costs (Alboofetileh et al. 2019).
Microwave-assisted extraction significantly reduces the extraction time and enhances the SP yield. Microwave radiation is used to heat the solvent and accelerate the extraction process. Furthermore, it is particularly advantageous for thermolabile compounds and has gained attention owing to its efficiency. However, precise control of the microwave parameters is essential to prevent polysaccharide degradation (Rodriguez-Jasso et al. 2011).
Ultrasound-assisted extraction is a non-thermal method that can reduce the environmental impact of solvent consumption. Furthermore, it is highly efficient in isolating thermosensitive compounds and results in a high yield with a shorter extraction duration. However, equipment costs and other physical factors, such as the matrix and structure of the sample may be limiting factors (Rodriguez-Jasso et al. 2011).
Supercritical fluid extraction uses a supercritical fluid as the extraction solvent under high temperatures and pressures. This method targets specific SPs and avoids the use of toxic solvents. However, the necessity of specific equipment and the initial costs are limitations of this method (Fabrowska et al. 2016).
Acid extraction methods typically use strong acids, such as hydrochloric or sulfuric acids, to hydrolyze the cell walls of seaweed and release SPs. This method results in a high yield and is suitable for specific applications that require controlled hydrolysis. However, product degradation and safety precautions are considered the limitations for industrial-scale production (Ale et al. 2011).
The ionic liquid-based extraction method utilizes an ionic solvent that can dissolve and extract SP efficiently. Furthermore, this method has gained attention for its minimum environmental impact and high-purity extracts. Nevertheless, ionic liquid extraction may necessitate the development of customized solvents and process optimization (Gereniu et al. 2018).
The selection of the most appropriate method for SP extraction from seaweed depends on various factors, such as cost considerations, specific requirements of the intended application, and environmental concerns. Furthermore, the global scientific community is continuously refining these methods to maximize the yield while maintaining the integrity and bioactivity of the extracted polysaccharides.
Characterization techniques of SPsThe characterization of SPs is essential for elucidating their structural properties, assessing their purity, and determining their suitability for various applications. This will lead to the discovery of the structure-activity relationship (SAR) and resolve future challenges. These techniques employ a range of analytical methods to provide comprehensive information on the molecular weights, compositions, conformations, and biological activities of SPs.
Fourier transform infrared spectroscopy is used to analyze the chemical bonds and functional groups present in the SP polymers. It assists in the identification of specific chemical groups such as sulfated esters and glycosidic linkages, which provide insights into the molecular structure of SPs (Amorim et al. 2012). Nuclear magnetic resonance (NMR) spectroscopy is another powerful tool for analyzing the detailed structural characteristics of SPs. This technique elucidates the glycosidic linkages, anomeric configurations, and sulfate substitution patterns. 1H-NMR and 1C-NMR have been widely used for this purpose (Sánchez et al. 2022). The molecular weight and structural features of the SPs are determined by using mass spectrometry. Currently, various mass spectrometry methods such as matrix-assisted laser desorption / ionization and electrospray ionization are commonly used (Bhardwaj et al. 2020). Further separation of the polymer based on various factors such as molecular weight ensures the accuracy of the characterization results. SPs can be distributed based on their molecular size, which helps determine the molecular weight distribution and provide information about their polydispersity. Widely used methods for this purpose are gel permeation or size-exclusion chromatography. High-performance liquid chromatography is another widely used separation and quantification technique that allows the quantification of specific sugar monomers in SPs and helps assess the compositional variations and molar ratios of the constituent sugars (Jaulneau et al. 2010). The three-dimensional structure and stability of the polymers are evaluated using circular dichroism spectroscopy. This technique aids in analyzing the secondary structure and conformational changes in SPs. The physical properties of the polymer, such as the morphology and microstructure of the particles are characterized by using imaging techniques such as scanning and transmission electron microscopy. Even then, the physical properties of SPs are only partially understood (Saravana et al. 2018). This understanding is further enhanced by employing X-ray diffraction, which is used to study the crystalline structure of the SPs to reveal information about the degree of crystallinity and arrangement of the polymer chains. Rheological analysis measures the flow and deformation behaviors of the SP solutions and gels. This characterization technique helps assess viscoelastic properties, which are important in applications such as food and cosmetics. Finally, the assessment of the functional activities of SPs, such as their anti-inflammatory, antioxidant, and anticoagulant properties, is vital. These assays provide crucial insights into potential therapeutic applications. Furthermore, the selection of characterization techniques depends on the specific properties and research objectives of the SPs under investigation. The complementary use of multiple techniques is often necessary to gain a comprehensive understanding of the structural and functional attributes of these complex molecules, enabling researchers to tailor their applications accordingly.
BIOLOGICAL ACTIVITIES AND HEALTH BENEFITS OF SULFATED POLYSACCHARIDESSPs isolated from seaweed have gained significant attention in recent years owing to their wide spectrum of biological activities and potential health benefits. SPs are primarily found in different seaweed categories including brown, red, and green. Their complex molecular structures consist of monosaccharide units that are chemically bound to sulfate groups and have a prominent effect on their biological activity, making them valuable candidates for numerous therapeutic applications. This section systematically elucidates their different primary biological activities, such as anticoagulant, antiviral, anti-inflammatory, and antioxidant activities, and their SAR that are crucial for determining their potential bioactivities. Furthermore, the potential implications of the use of SPs for diverse medical conditions are examined.
Anticoagulant and antithrombotic propertiesAnticoagulant and antithrombotic activities are offered by different SPs, such as by the heparin-like compounds found in brown seaweed. They can interfere with blood clot formation, making them potential therapeutic agents for cardiovascular diseases (Costa et al. 2010). The degree of sulfation is a fundamental property used to evaluate anticoagulant activity. A higher degree of sulfation offers a strong negative charge to the polymer, which leads to interactions with positively charged components in the blood-clotting cascades, including thrombin factor Xa, through electrostatic interactions. This can be affected by the distribution of sulfated groups along the polysaccharide chain. This specific arrangement of sulfate groups provides accessibility for binding to blood-clotting factors. A higher sulfate density evenly distributed along the chain enhances anticoagulant properties (Ciancia et al. 2010). Moreover, specific sugar motifs such as uronic acid and fucose aid in the generation of binding sites on the polymer for clotting factors and contribute to the overall anticoagulant activity. Furthermore, this interaction affects the length and three-dimensional structure of SP. Longer polymers and extended structures provide more binding sites for clotting factors and their enhance anticoagulant activity. The sulfation pattern also plays a significant role in SP activity. The arrangement of neighboring sulfate groups can affect the ability of the molecule to bind to clotting factors.
Similar to anticoagulant activity, the degree of sulfation plays a role in antithrombotic activity. A more negatively charged polysaccharide offers greater interaction with clotting factors and platelets, which leads to reduced thrombotic risk. The density and distribution of sulfate groups also play important roles in the antithrombotic activity. Distribution of sulfates is crucial for their consistent antithrombotic effects. Furthermore, sugar motifs, such as glucuronic acid, play an important role in the antithrombotic effect by providing binding sites for clotting factors and platelets. SPs bind to the surface receptors of platelets through electrostatic interactions, which interfere with platelet aggregation by preventing them from adhering to each other or to the vascular endothelium. Moreover, SPs reduce fibrin clot formation by inhibiting the conversion of fibrinogen to fibrin, thereby enhancing their antithrombotic activity. SPs also enhance thrombus resolution, aiding in the breakdown of existing clots. This effect is mediated by their interactions with plasminogen and tissue plasminogen activator (Ciancia et al. 2010).
Anti-inflammatory propertiesThe distribution and density of sulfate groups in a polymer are key factors in its anti-inflammatory activity. Higher sulfation provides more negatively charged molecules that interact with proinflammatory molecules, and the even distribution of these sulfate groups provides a consistent anti-inflammatory potential. Sugar composition is another important factor responsible for anti-inflammatory effects. Specific sugar motifs such as mannose, glucuronic acid, and fucose interact with inflammatory mediators and significantly contribute to their anti-inflammatory potential. The affinity of each SP to key inflammatory proteins is significantly affected by its sulfate pattern. The arrangement of sulfate groups on sugar units, including the position and proximity of neighboring sulfates, leads to anti-inflammatory effects (Jayawardena et al. 2022, Lee et al. 2022). Previous studies have shown that the molecular weight of SPs plays an important role in their anti-inflammatory properties. Smaller fragments may have enhanced bioavailability and better tissue penetration, leading to more effective interactions with the inflammatory mediators (Nagahawatta et al. 2022). SP contains a specific pattern of sulfation and branching that influences its binding to cell receptors, leading to the regulation of signaling pathways. The location of sulfate groups and branching points must be carefully considered to maximize the anti-inflammatory effects. The conformation, size, and overall charge of polysaccharides are important factors that affect their affinity to inflammatory mediators. Larger molecules may have more extensive binding sites, whereas charge distribution affects the electrostatic interactions with inflammatory factors. SPs regulate immune responses by influencing the production of proinflammatory and anti-inflammatory cytokines. Achieving a balance between these responses is critical for preventing excessive inflammation and immune suppression. Most studies regarding the anti-inflammatory activity of SPs exhibit the inhibition of nuclear factor кB (NF-κB) signaling pathway (Park et al. 2011, Wu et al. 2016, Jayawardena et al. 2020b). Understanding how SPs interfere with this pathway is essential for optimizing their anti-inflammatory potential. Furthermore, optimization of the structural features of SPs can lead to the development of potent anti-inflammatory agents with therapeutic applications. Further studies should be conducted to determine these structural attributes to target specific inflammatory pathways and achieve the desired anti-inflammatory outcomes while minimizing the potential side effects.
Antiviral propertiesAs mentioned previously, the density and distribution of sulfate groups along the polysaccharide chain are crucial for their antiviral activity. Higher sulfate density provides more negatively charged sulfate groups that interact with viral surface proteins. An even distribution ensures a consistent antiviral effect. Moreover, sugar composition and sulfate patterns also play a significant role in the affinity with viral surface proteins. Specific sugar motifs, such as sulfated motifs, and their arrangement, such as the position and proximity of neighboring sulfates, affect the affinity of the polymer for viral attachment proteins (Lu et al. 2021). As mentioned earlier, molecular weight is crucial for antiviral activity. Smaller fragments may have enhanced their bioavailability and better tissue penetration, leading to more effective interactions with viral particles. Another application of SPs as antiviral agents is to mimic the host cell receptors. This prevents viral attachment to host cell receptors and cell entry mechanisms. Furthermore, some SPs interfere with the viral envelope proteins and inhibit cell entry by preventing viral fusion with host cell receptors (Hans et al. 2021, Wei et al. 2022). Some studies have reported that SPs interfere with different stages of the viral life cycle and inhibit viral replication in host cells (Wei et al. 2022). In addition, SPs enhance host immune responses against viral infections (Huang et al. 2019). Studying how they interact with the host immune system and modulate immune responses is crucial for future development of antiviral strategies.
Antioxidant propertiesThe degree of sulfation plays a pivotal role in the antioxidant activity because of its electron donation potential, which can neutralize free radicals. Similar to showing biological activities, lower molecular weight SP fragments show higher antioxidant activities owing to their elevated accessibility to free radicals and reactive oxygen species (Wang et al. 2008, Barahona et al. 2011). However, this factor requires further understanding for better application purposes. Specific sugar motifs in polymers, such as xylose and mannose, which have a higher ability to donate electrons, are crucial for their radical scavenging ability. The density and pattern of sulfates in the polymer are also important for their antioxidant capacity. Higher sulfate density and a specific pattern of distribution enhance the ability of molecules to access reactive species and neutralize free radicals. In addition, some SPs exhibit antioxidant potential by chelating metal ions, such as iron and copper, which can catalyze oxidative reactions. This leads to a decrease in the formation of harmful radicals (Wang et al. 2008). Understanding the specificity and affinity of metal ions is crucial for optimizing this mechanism. The chain length of SPs is also positively correlated with their antioxidant ability. However, this was affected by the other factors mentioned earlier. SPs can interact synergistically with other antioxidant drugs or compounds to enhance their activity. Understanding these potential interactions is important for the development of effective combinations of antioxidants. Recognizing the structural features of SPs and their influence on their antioxidant activities is crucial for their therapeutic application, and further research must be conducted to understand the structural attributes that maximize the antioxidant effects while considering safety and biocompatibility.
Immunomodulation propertiesThe sizes and molecular weights of SPs significantly affect their immunomodulatory potential. Some studies have reported that smaller fragments may exhibit enhanced immunomodulatory properties because they can penetrate tissues more readily and effectively engage immune receptors (Karnjanapratum and You 2011, Wijesinghe et al. 2012). Some studies have reported that molecules with high molecular weights show enhanced immunomodulatory activity (Yoo et al. 2019, Apostolova et al. 2020). Therefore, researchers must carefully tailor these structural attributes to target the immunomodulatory effects of SPs. Similar to the other aforementioned activities, sulfate density, sulfate pattern, and branching within the polysaccharide structure maximize its immunomodulatory activity by providing more sites for binding to immune receptors and cytokines that influence the interaction of SP with immune cells and molecules. Specific sugar motifs, such as fucose, mannose, and glucuronic acid, may interact with immune cells and regulate their immune response. The binding specificity of SPs for immune receptors, cytokines, and chemokines is pivotal for their immunomodulatory potential. Further investigation and understanding of precise binding targets and mechanisms are essential for designing effective immunomodulators. Moreover, SPs have the potential to activate immune cells such as macrophages and dendritic cells (DCs). This leads to the activation of cytokine production in macrophages, which are essential for immunomodulation. SPs may fine-tune immune responses and prevent excessive inflammation or immune suppression by modulating the immune cell signaling. Understanding these regulatory mechanisms is crucial for their therapeutic applications. Further, some studies have mentioned that SPs potentially stimulate the immune cells against different infectious diseases (Apostolova et al. 2020). Understanding how these molecules interact with the host immune system and modulate immune responses is vital for the development of comprehensive immunomodulation strategies.
Other than these major biological activities, SPs exhibit various health benefits that have commercial and industrial value. The SAR between those biological activities and the structures of SPs were summarized in Table 1.
POTENTIALS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONSeaweed-derived science parks have gained global recognition owing to their versatile applications across multiple industries. The commercial significance of each SP depends on its specific composition and bioactivity, which can vary with seaweed conditions, harvesting season, and environmental factors (Nagahawatta et al. 2021, Jiksing et al. 2022, Yang and Kim 2022).
Commercial significance of SPs isolated from brown seaweedBrown seaweed are diverse groups of marine macroalgae that contain brownish or olive-green pigments, such as fucoxanthin. SPs isolated from brown seaweed are prized for their versatility, biocompatibility, and sustainability. Their wide range of applications continues to expand through continuous investigation and exploration. Furthermore, their natural origin, low risk of resistance, and broad range of activities are advantages over some currently available drugs, such as warfarin and anticoagulant, with delayed onset of action and requiring frequent monitoring of blood levels. However, SP-like heparin provides rapid onset of action without constant monitoring.
Fucoidans, a major type of SP isolated from brown seaweed, have a wide spectrum of commercial and industrial applications because of their specific structural features. Recent advances in chromatographic techniques have allowed for the deep biochemical characterization of various fucoidanases and sulfatases. This has resulted in the identification of their monomeric compositions, sulfation patterns, and branching sites. The fucoidan backbone consists of various monosugars, resulting in different fucoidan backbones such as sulfated fucans (F-fucoidans), galactofucans / fucogalactans, fucomannoglucuronans (GA or U-fucoidans), and (G-fucoidans or G-fucans). The identified chemical backbones are summarized in Fig. 2 (Zayed et al. 2022).
Fucoidans are widely used in pharmaceutical industries as they exhibit anticoagulant and antithrombotic properties through the inhibition of blood clot formation. They have also been identified as alternatives to heparin, an anticoagulant derived from animal tissues. Mauray et al. (1995) investigated the activity of fucoidan isolated from the brown seaweed Ascophyllum nodosum. Fucoidan significantly enhanced thrombin inhibition by antithrombin and heparin cofactor II by increasing its effectiveness compared with uncatalyzed reactions. Furthermore, it exhibited strong antithrombotic effects in a rabbit model of venous thrombosis, with an effective dose (ED80) of 1.8 mg kg−1 by inhibiting thrombus formation by 80%, which is comparable to that of heparin. Notably, the antithrombotic effect of fucoidan lasts longer than that of heparin and significantly prolongs the thrombin clotting time. However, there was a slight increase in the bleeding time after fucoidan infusion at ED80. The results of the present study suggest the potential of fucoidan as an antithrombotic drug. Further clinical trials are required to confirm its safety and efficacy (Mauray et al. 1995). Another study on the antithrombotic activity of orally administered low-molecular-weight fucoidans isolated from Laminaria japonica exhibited specificity in inhibiting thrombin-induced platelet aggregation and promoting tissue factor pathway inhibitor, potentially making it a safer antithrombotic agent than aspirin (Zhao et al. 2016). A study on fucoidan-loaded nanoparticles revealed that 1% chitosan-fucoidan nanoparticles with glutaraldehyde crosslinking showed promising characteristics for oral anticoagulant therapy. These nanoparticles exhibited resistance to gastric pH, enhanced in vitro anticoagulant activity, improved oral antithrombotic effects, and created a safe hemorrhagic profile, highlighting the previously unexplored oral antithrombotic profile of commercially available fucoidan from Fucus vesiculosus and opening new possibilities for its application (da Silva et al. 2018).
Fucoidans are well known for their anticancer activities as confirmed by various studies. They show inhibitory potential against tumor growth and metastasis and enhance the effectiveness of chemotherapeutic drugs. Choi and Kim (2013) confirmed the anticancer potential of a low-molecular-weight fucoidan isolated from F. vesiculosus by inhibiting cell transformation. Moreover, Palanisamy et al. (2017) reported the anticancer activity of fucoidan isolated from Sargassum polycystum by inhibiting the growth of the MCF-7 human breast cancer cell line, accompanied by cytomorphological changes and induction of apoptosis in fucoidan-treated cells. This anticancer activity was further confirmed in vitro and in vivo by Lin et al. (2020). The results of this study revealed that fucoidan exerts direct anticancer effects by inducing cell cycle arrest, apoptosis, and other mechanisms. Furthermore, they indirectly combat cancer by activating natural killer cells, macrophages, and other components of the immune system to target cancer cells. Notably, this study suggested a synergistic effect of fucoidan and a standalone antitumor drug. It is valued for its strong biological activity, widespread availability, and low susceptibility to drug resistance, and minimal side effects (Lin et al. 2020).
Another important pharmaceutical use of fucoidan is as an anti-inflammatory and immunomodulatory agent. The anti-inflammatory potential of fucoidans isolated from brown seaweed has been extensively studied (Jayawardena et al. 2020a, 2020b, 2022, Nagahawatta et al. 2022) and the role of SP structure in anti-inflammatory and immunomodulatory activities has been previously noted. Mainly, fucoidan inhibits surface receptors such as toll-like receptors and interferes with NF-κB with and MAPK pathways. Fucoidan mainly inhibits the phosphorylation of proteins in these signaling pathways and downregulates the gene and protein expression of proinflammatory cytokines. These results were further confirmed by Manikandan et al. (2020) through an in vivo evaluation of fucoidans isolated from Turbinaria decurrens. They revealed that fucoidan reduced paw swelling in mice with formalin-induced inflammatory edema. Further, fucoidan administration resulted in the retention of p65/NF-κB transcription factor in the cytosol, leading to downregulation of the gene expression of proinflammatory mediators such as interleukin (IL)-1β, cyclooxygenase-2 (COX-2), and matrix metalloproteinase 9 (Manikandan et al. 2020).
The immunomodulatory activity of fucoidans isolated from F. vesiculosus showed that rats treated with a single dose of aspirin (400 mg kg−1) showed significant alterations in various parameters, including the levels of total nitrite and nitrate, interleukins (IL-4, 6, 10, 12), tumor necrosis factor-α, and interferon-γ. They also exhibited collagen deposition in the glandular tissue and changes in the localization of cyclooxygenases 1 and 2 and epidermal growth factor. Rats pretreated with fucoidan (0.02 g kg−1 d−1 for two weeks orally) showed significant prevention of the above-mentioned alterations and damage induced by aspirin. This study suggests that fucoidan exhibits gastroprotective properties against aspirin-induced gastric mucosal damage in rats. Furthermore, fucoidan demonstrates promise as a potential therapeutic agent for mitigating gastric ulcers and related complications caused by nonsteroidal anti-inflammatory drugs such as aspirin. Fucoidans isolated from S. hemiphyllum exhibited excellent immunostimulatory activity by significantly increasing nitric oxide (NO) secretion in macrophage RAW 264.7 cells. This increase in the NO secretion was associated with the upregulation of COX-2 and inducible nitric oxide synthase at both gene and protein abundance levels (Li et al. 2022). Oral administration of a fucoidan isolated from Undaria pinnatifida resulted in remarkable immunomodulatory activities in sarcoma 180 subcutaneous xenograft-bearing mice. It enhanced the phagocytotic function of the mononuclear phagocytic system and increased the serum levels of Th1 cytokines, including IL-2, interferon-γ, and tumor necrosis factor-α. Further, fucoidan promoted splenic T lymphocyte responses and increased the infiltration of CD11c+ DCs into transplanted sarcoma tissues. Finally, it upregulated the surface expression of MHC class II and the costimulatory molecule CD86 on bone marrow-derived CD11c+ DCs in S180-bearing mice (Li et al. 2023).
Fucoidans are also used in skincare products due to their moisturizing, antioxidant, and aging-related properties. They help improve skin hydration and reduce the signs of aging. The hydration and soothing properties of fucoidan have led to the development of fucoidan-based face masks. They are used for pampering and revitalizing the skin. Moreover, they possess anti-inflammatory properties that soothe and calm the skin and aid in the treatment of acne. Fucoidan particles can be used as exfoliating agents in scrubs and facial cleansers. They provide gentle exfoliation and help remove dead skin cells and impurities from the skin surface. Furthermore, fucoidans can be found in hair care products, such as shampoos and conditioners, to enhance hair health. Furthermore, some fucoidans exhibit UV-absorbing properties that are valuable for the development of sunscreen and UV protection products. These formulations help to shield the skin from harmful UV radiation and reduce the risk of sunburn. Wang et al. (2020) reported the anti-photoaging and anti-melanogenic effects of fucoidan, with a molecular weight of 102.67 kDa from Hizikia fusiformis, against UVB-induced human keratinocytes and B16F10 melanoma cells stimulated with alpha-melanocyte-stimulating hormone. Fucoidan successfully inhibited UVB-induced reactive oxygen species levels and the expression of related proteins (Bax, Bcl-xL, poly[ADP-ribose] polymerase, and Caspase-3). Furthermore, it reduced the expression of melanogenesis-related proteins (tyrosinase, tyrosinase related protein [TRP]-1, and TRP-2) and microphthalmia-associated transcription factor through regulation of the ERK-MAPK pathway pathway (Wang et al. 2020). Another study produced AgNPs coated with chitosan and fucoidan, which exhibited strong antimicrobial effects that were useful for cosmeceutical applications (Venkatesan et al. 2018).
Fucoidans are valuable to the food industry owing to their potential health benefits, including immune support and antioxidant properties, functional properties, and nutritional value. They are incorporated into functional foods such as energy bars and health drinks to enhance their nutritional value. They are also used as dietary supplements and are available in various forms such as tablets, capsules, and powders. These products make fucoidan easily accessible and easily incorporable into consumers’ daily diets. Moreover, fucoidan can be added to beverages such as smoothies and fruit juices to increase their nutritional value and functional properties. Seaweed-based sacks are another valuable product containing fucoidan and provide dietary fiber and essential minerals. Apart from these products, fucoidans are incorporated into weight management as prebiotics and natural flavor enhancers. The nutritional and functional value of fucoidans has been confirmed in numerous studies (Shen et al. 2018, Zhao et al. 2018, Fitton et al. 2019, Anisha et al. 2022).
Commercial significance of SPs isolated from red seaweedRed seaweed is a diverse group of marine macroalgae characterized by its red or purplish color, which results from the presence of pigments such as phycoerythrin and phycocyanin. The SPs isolated from red algae exhibit commercially valuable biological activities. Galactans are the most abundant SP in red seaweed and are commercially important because of their remarkable gelling and thickening properties. They can be divided into two main groups depending on their stereochemistry—agarans and carrageenans. Galactans with 4-linked α-galactose residues of the l-series are known as agarans, and galactans with the d-series are known as carrageenans.
Carrageenans are widely and frequently utilized SPs in various industries including in the food industry as a gelling and thickening agent in various products, such as dairy desserts, ice creams, and jellies, to provide texture and stability. Furthermore, they serve as a stabilizer in dairy products, preventing ingredient separation in products such as chocolate milk, and ensuring a smooth and creamy texture in ice cream (Blakemore and Harpell 2009, Imeson 2009, Campbell and Hotchkiss 2017). Hotchkiss et al. (2016) highlighted the significance of carrageenan as a leading seaweed product in the above-mentioned industries and pointed out that carrageenan holds the fourth largest share in the global food texture market, following starches, gelatin, and pectin, based on value. Furthermore, hydrocolloids such as carrageenan are widely used in the global market as healthier foods to reduce unhealthy ingredients such as salt, sugar, and fat (Hotchkiss et al. 2016). A safety study conducted by Weiner (2014) revealed that carrageenans are non-carcinogenic and non-genotoxic in animal studies. Furthermore, the safety of carrageenan in infant formulas has been confirmed through epidemiological studies of infant baboons and human infants through epidemiological studies (Weiner 2014). Carrageenans are used as emulsifiers in salad dressings and sauces to prevent separation of oil and water. Furthermore, they are useful in the meat and poultry industry for improving texture and reducing cooking loss. They are commonly utilized in plant-based milk alternatives such as coconut and almond milk to enhance their creaminess and mouthfeel. They are also used in canned pet foods to enhance texture and palatability (Blakemore and Harpell 2009).
Carrageenans are widely produced in the pharmaceutical industry because of their natural origin and unique properties, including gelling, thickening, and stabilizing abilities. They are used in oral drug formulations (syrups and suspensions) as a suspension and thickening agent. They are also employed in wound care products such as dressings and topical gels to improve the moist environment and prevent infections to promote wound healing. These emollient and moisturizing properties of carrageenans have gained applications in dermatological products, such as creams and ointments. They help treat dry skin and eczema by soothing and hydrating the skin. Carrageenans are included in nasal sprays and inhalers as they provide a protective layer to avoid irritation of the mucous membrane and improve drug delivery. The gelling and stabilizing properties of carrageenans are useful in improving the effectiveness of dental hygiene products, such as dental gels and mouthwashes. According to Weiner (2014) ingested carrageenans are excreted through feces without significant degradation from low gastric pH or the gastrointestinal microflora (Weiner 2014). This specific characteristic aids the development of gastrointestinal drug delivery systems based on carrageenans. The carrageenan coat protects the drugs from gastric acids and ensures targeted delivery to the gastrointestinal tract. These properties of carrageenans are utilized to produce easy-to-administer gel capsules and eye drops with improved viscosity and retention time on the ocular surface. In addition to these pharmaceutical applications, carrageenans are used as an imaging agent in medical imaging procedures such as magnetic resonance imaging and computed tomography. Furthermore, they are useful in tissue engineering applications to support cell growth and tissue regeneration. The galactan backbone of carrageenans is thought to be generated in Golgi bodies, whereas sulfation occurs in the cell wall by sulfotransferases (Therkelsen 1993). The possible biosynthetic pathways and major structures of carrageenans are summarized in Fig. 3.
Commercial significance of SPs isolated from green seaweedGreen seaweed exhibit green pigmentation caused by chlorophyll and other pigments. They play pivotal roles in marine ecosystems, including coastal areas and coral reefs. Ulvans are the major SPs isolated from green seaweed owing to their wide spectrum of commercial value. Although scientific studies are ongoing, some of their unique properties are being utilized in current industries. Ulvans have properties considered valuable for the food industry. They are used as gelling, thickening, and stabilizing agents in various products. Generally, ulvans consist of glucuronic acid (22.5%), sulfated rhamnose (45%), xylose (9.6%), and iduronic acid (5%) linked with α- and β-(1,4)-glycosidic linkages (Muthukumar et al. 2021). Ulvans contain two major disaccharide units, ulvanobiuronic acid or aldobiuronic types A and B (Fig. 4A), and ulvanobioses with type U. Among these disaccharides, aldobiuronics are more common (Fig. 4B). Therefore, the presence of boric acid, calcium, or divalent ions facilitates the generation of weak gels. However, the underlying mechanism has not been fully elucidated. Lahaye and Robic (2007) proposed a mechanism that describes the generation of crosslinks in ulvan chains. To crosslink the ulvan chains, a few borate esters were generated with the cis-diol functionalities of the unsulfated rhamnose residues (Lahaye and Robic 2007) (Fig. 4C).
Ulvans are utilized in jellies, jams, and confectionery items as a gelling agent, and they can be used as an alternative source of traditional thickening agents, such as carrageenan and agar. Furthermore, their thickening ability is used to improve the texture and consistency of food products such as soups, sauces, and gravies (Anisha et al. 2023). The utilization of fucans in the functional food industry as dietary fibers to improve nutritional value and digestive health is vital (Anisha et al. 2023). Some green seaweed species exhibit antimicrobial activities. According to Tran et al. (2018) ulvan isolated from Ulva reticulate composed of rhamnose, galactose, xylose, mannose, and glucose at a 1 : 0.12 : 0.1 : 0.06 : 0.03 ratio, showed remarkable antimicrobial activities by inhibiting Enterobacter cloacae and Escherichia coli with 20 mm and 18 mm inhibition zones, respectively (Tran et al. 2018). Another study reported the antimicrobial activity of ulvan isolated from U. lactuca against both human and fish pathogens. Furthermore, they developed an ulvan chitosan hydrogel that exhibited enhanced antimicrobial activity (Ibrahim et al. 2022). This property is useful in the food preservation industry for extending the shelf life of perishable foods. Moreover, these specific applications enhance the texture, stability, moisture retention, and shelf life of the products in the bakery (bread and pastries), dairy (yogurt and deserts), and beverage (fruit juices, smoothies, and protein drinks) industries. Ulvans may contribute to the umami flavor profile of food products, making it an interesting ingredient in savory dishes and seasonings.
Apart from the antimicrobial activity of ulvans, they show various biological activities useful in the pharmaceutical industry, such as antioxidation, antithrombin, anti-inflammation, immunomodulatory, and anticancer activities (Flórez-Fernández et al. 2023). According to Li et al. (2018), ulvan isolated from U. pertusa showed remarkable antioxidant activity. Further, results of this study exhibited a purified ulvan sample with high uronic acid and sulfate content and a relatively low molecular weight, demonstrated potent antioxidant activity by reducing malondialdehyde levels and increasing superoxide dismutase and catalase levels in the liver (Li et al. 2018). The anti-inflammatory effects of ulvan isolated from U. fasciata (Moawad et al. 2022), U. lactuca (de Araújo et al. 2016), and U. rigida (Kikionis et al. 2022) have solidified their potential in the pharmaceutical industry as a target agent against oxidative stress and inflammation-related conditions. Kidgell et al. (2020) reported the immunomodulatory potential of ulvan isolated from U. ohnoi. Higher molecular weight ulvan fractions at 100 μg mL−1 demonstrated an anti-inflammatory effect by reducing the secretion of IL-10 and prostaglandin E2 (Kidgell et al. 2020). This activity was confirmed in another study that determined the immunomodulatory activity of ulvan saccharides (Zhang et al. 2020). These activities, along with the antimicrobial activities, aid in the production of wound care products such as hydrogels and dressings. Ulvans have been utilized in the development of drug delivery systems, such as microspheres and nanoparticles. Tziveleka et al. (2022) developed an ulvan-coated antibacterial drug-delivery platform using U. rigida (Tziveleka et al. 2022). Fernández-Díaz et al. further confirmed this by incorporating ulvan isolated from U. ohnoi into nanoparticles (Fernández-Díaz et al. 2017). These systems can encapsulate pharmaceutical compounds, protect them from degradation, and enable their controlled release. Furthermore, ulvan-based formulations have been used for oral drug delivery to improve the solubility and bioavailability of poorly water-soluble drugs. In addition, ulvans are used as stabilizers and thickeners in pharmaceutical formulations and dental products, such as mouthwashes. Ulvans play an important role in biomedical studies, including cell culture, as substrates or scaffolds for various cell types, tissue engineering, and the development of scaffolds for regenerative medicine. Ulvan extracted from U. pertusa showed remarkable antiviral activities against vesicular stomatitis virus. Ulvan with a molecular weight of 1,068.2 kDa and ulvan-F1 with a molecular weight of 38.5 kDa exhibited significant inhibitory effects on the infection and replication of vesicular stomatitis virus at a concentration of 100 μg mL−1. The rates of inhibition of the virus replication were 40.75% for ulvan and 40.13% for ulvan-F1 (Chi et al. 2020). Ulvan from U. lactuca exhibited antiviral efficacy against the hepatitis A virus strain 10 (Maray et al. 2023). Further studies have shown that ulvan isolated from Enteromorpha compressa and U. intestinalis showed significant antiviral activities against the herpes simplex and measles virus by inhibiting the adsorption and replication of the virus and by reducing syncytia formation and low cytotoxicity, respectively. Therefore, ulvans are considered potent antiviral agents. In addition to ulvan, rhamnan sulfate, isolated from the green seaweed Monostroma nitidum exhibits antiviral activity against SARS-CoV-2 (Song et al. 2021). Rhamnan sulfate, isolated from M. latissimum, showed remarkable antithrombin activity, similar to that of heparin. Furthermore, this study showed that the rhamnan sulfate isolated from M. latissimum was significantly different from that isolated from ulvan. Furthermore, sulfated groups are mainly attached to the C-3 or C-4 positions of 1,2-linked rhamnose residues, which can significantly affect their biological activities (Lee et al. 1998).
As discussed in the previous section regarding the SAR between SP and their biological activities, the structural features of these polymers are crucial for determining their potential bioactivities that are valuable for industrial applications. Table 2 shows the various SPs and their biological activities and structural features. These monomeric structures combined with sulfate groups form a complex molecular landscape that is crucial for biological activities. Understanding this actual structural makeup using SAR knowledge will lead to further elucidation of valuable insights into the mechanisms that exert their beneficial effects.
CHALLENGES AND FUTURE DIRECTIONSThe exploration of multifaceted SPs isolated from seaweed revealed that they inevitably encounter promising opportunities as well as significant challenges. Mainly, the complexity and variability of these SPs are responsible for their immense potential biological activities in a wide spectrum. Therefore, addressing these challenges offers a future direction in this field through discoveries and innovations (Knoop et al. 2022).
Variability of compositionSignificant variability in composition is considered a major issue observed among and within different seaweed species. This variability can be caused by several factors such as environmental conditions, geographic location, and growth stage (Salehi et al. 2019). Different seaweed species such as Phaeophyta, Rhodophyta, and Chlorophyta typically contain fucoidan, carrageenan, and ulvan as their major SP. Different polysaccharide profiles are observed in each species. Environmental variables, such as water temperature, salinity, nutrient variability, and sunlight exposure, can significantly affect seaweed chemical composition. These factors can vary in different geographical locations, resulting in science parks with distinct structures and properties. Seasonal variations and growth stages of seaweed also play important roles in the chemical profiles of SP. Intraspecific variabilities due to genetics, local habitat, and growth stage are also responsible for variations in the content and structure of SP (Piriz et al. 2003). Meanwhile, SPs exhibit structural diversity; for example, fucoidan isolated from brown seaweed shows different branching patterns, molecular weights, and degrees of sulfation, which significantly affect its biological activity (Ale et al. 2011).
The variability in composition raises challenges for utilizing SP on an industrial and commercial scale because there is no available universal approach. Therefore, it is essential for researchers to carefully characterize specific SPs when designing experiments and applications. Furthermore, developing a standardized method for isolation and characterization that avoids these challenges is valuable for industries and is helpful for increasing their utilization.
Extraction and purificationThe extraction and purification of SP from seaweed are crucial steps for the utilization of their biological activities. However, this process can be challenging and complex because of several factors. Seaweed diversity is the dominant factor (Costa et al. 2010). The selection of the extraction method depends on the seaweed species used to yield a high quality and quantity of SP. Matrix complexity is another factor affecting the extraction method. Seaweed contain varying amounts of carbohydrates, proteins, lipids, and other compounds. Therefore, extraction methods should selectively target SP to separate them from the matrix while minimizing the co-extraction of unwanted substances. The yield and purity of SPs are also important factors in their isolation. Some extraction methods may yield higher quantities of SPs, but with lower purity, whereas others may prioritize purity with lower yields (Ale et al. 2011). Therefore, balancing these factors is essential to develop isolation methods for commercial applications. The quality and quantity of SP are highly affected by a lack of standardization methods. Scientists must focus on developing standardized methods that are significant at the industrial scale to maintain product quality. However, some extraction methods cause serious environmental issues because they require harsh chemicals and high temperatures. Therefore, developing a sustainable method for SP extraction is crucial. The scalability of extraction methods is another pivotal factor because of the applicability of SP in various industries, such as pharmaceutical and functional foods. The most important factor at the commercial scale is the cost-efficiency of the extraction method. Therefore, the development of cost-efficient techniques is crucial to make these compounds accessible to numerous industries. Finally, accurate analysis and quantification of SPs are pivotal research and quality control methods. Further studies are needed to develop analytical techniques that are robust and capable of handling the complex structures of these compounds. To address these challenges, researchers are continually exploring novel extraction techniques, improving existing methods, and developing standardized protocols with sustainable practices that consider the environmental impacts of extraction.
Diversity of biological activitiesSPs exhibit remarkable biological activities over a wide range. However, this diversity poses several challenges such as multifunctionality. Some SPs exhibit anti-inflammatory, antiviral, and anticoagulant activities (Pradhan et al. 2020). This multifunctionality makes it challenging to pinpoint specific mechanisms of action and tailor their uses for specific applications. This affects the quantification of the biological activities of SPs. Therefore, scientists are encouraged to develop standardized protocols, relevant assays, and endpoints. Understanding SAR is vital for its application. Variations in sulfation patterns, monosaccharide composition, molecular weight, and other structural features can result in different bioactivities, even within the same species. Therefore, intensive studies are required to better understand the SAR of SPs. Some SPs have specific targets for their biological activities (Ortega-Barria and Boothroyd 1999). Therefore, determining the specificity and selectivity of these compounds for biological processes is crucial for optimizing their commercial use. Various studies, such as those by Delma et al. (2014) and Morán-Santibañez et al. (2016) have emphasized the synergistic and antagonistic effects on overall biological activity. The identification of these interactions and their implications is vital for optimizing potential drug-drug or drug-food interactions for medical applications (Delma et al. 2014, Morán-Santibañez et al. 2016). The transition from promising in vitro and preclinical studies to clinical application is challenging. Demonstrating the safety and efficacy of SPs in human trials, particularly for the treatment of complex diseases, requires rigorous research and regulatory approval. Furthermore, establishing dose-response relationships, especially in therapeutic contexts that balance efficacy with safety, is a critical consideration.
Regulatory approval, consumer acceptance, and intellectual propertyRegulatory approval is a significant challenge in the application of SPs. This challenge encompasses several key aspects, as summarized in Table 3. Consumer acceptance is also a crucial challenge, especially in industries such as food, cosmetics, and nutraceuticals, which are the major industries that convert SPs into consumer products. These can be divided into nine major categories, as summarized in Table 3. Intellectual property is also an important aspect of the research, development, and commercialization of SPs. It consists of patents, trademarks, copyrights, and trade secrets that protect the innovations, discoveries, and formulations of SPs. The factors that should be considered for intellectual property are summarized in Table 3.
CONCLUSIONIn conclusion, this review provides a comprehensive overview of the functions and value of SPs derived from seaweed. SPs have gained significant attention owing to their remarkable biological activity against a wide spectrum of diseases. Therefore, they are valuable assets with multifaceted functions and considerable potential in various sectors. The SAR analysis of SPs provides insights into the utilization of SPs for commercial applications that have a significant impact on human health, industry, and innovation. Furthermore, the commercial applications of these compounds in brown, red, and green seaweed provide considerable knowledge regarding their value as natural compounds. However, the journey of SP from seaweed to the market involves numerous challenges that need to be addressed. The extraction and purification processes, as well as the variability in composition among the same and different seaweed species, result in significant hurdles that need to be overcome. Scientists and industry stakeholders should develop standardized methods to ensure consistency and scalability. The diversity of the biological activities requires further investigation and elucidation for better applications. One of the most critical aspects of harnessing the potential of SPs is navigating the regulatory approval process. Safety and efficacy data must be rigorously generated and submitted to regulatory authorities for evaluation.
ACKNOWLEDGEMENTSThis work was supported by the Qingdao International Innovation Cooperation Project for Science and Technology (No. 22-3-6-ghgg-1-hz).
Fig. 2Different fucoidan backbones isolated from brown seaweeds. (A) Sulfated fucans isolated from Lessonia spp. (B) Sulfated galactofucans isolated from Hormophysa cuneiformis. (C) Fucoidan containing uronic acid at O-2 isolated from Cladosiphon okamuranus. (D) Sulfated xylofucan from Punctaria plantaginea. 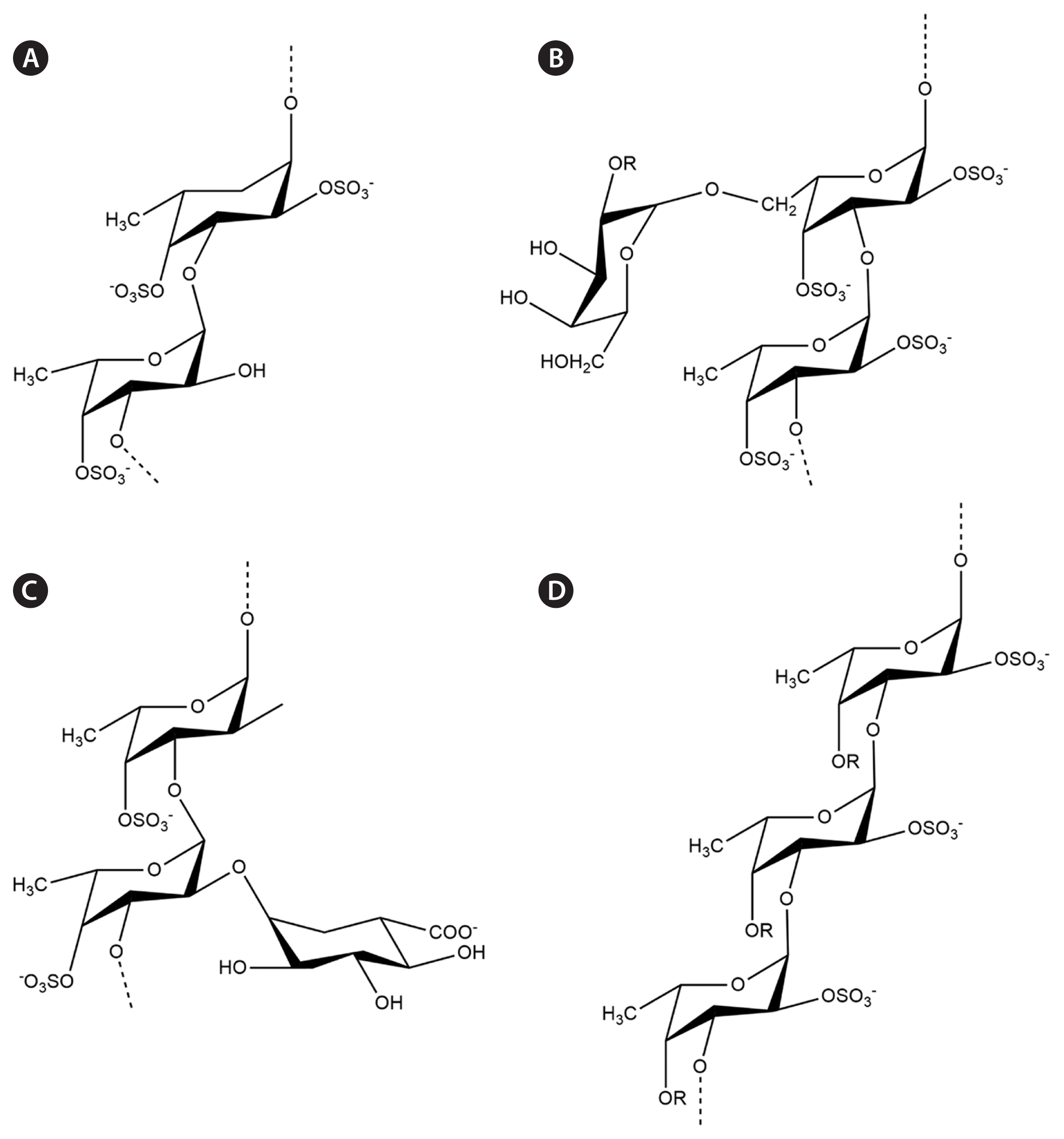
Fig. 3Biosynthesis of carrageenan. (A) Possible pathways and (B) synthesis of carrageenan from red seaweeds by treating OH−. S”t”ase, sulfatetransferase; 4-Gal, 4-O-substituted a-D-galactopyranosyl unit; 3-Gal, 3-O-substituted / 3-D-galactopyranosyl unit; Lambda, lambda (λ) carrageenan; Mu, Mu (μ) carrageenan; KAPPA, Kappa (κ) carrageenan; Nu, Nu (ν) carrageenan; IOTA, Iota (ι) carrageenan. 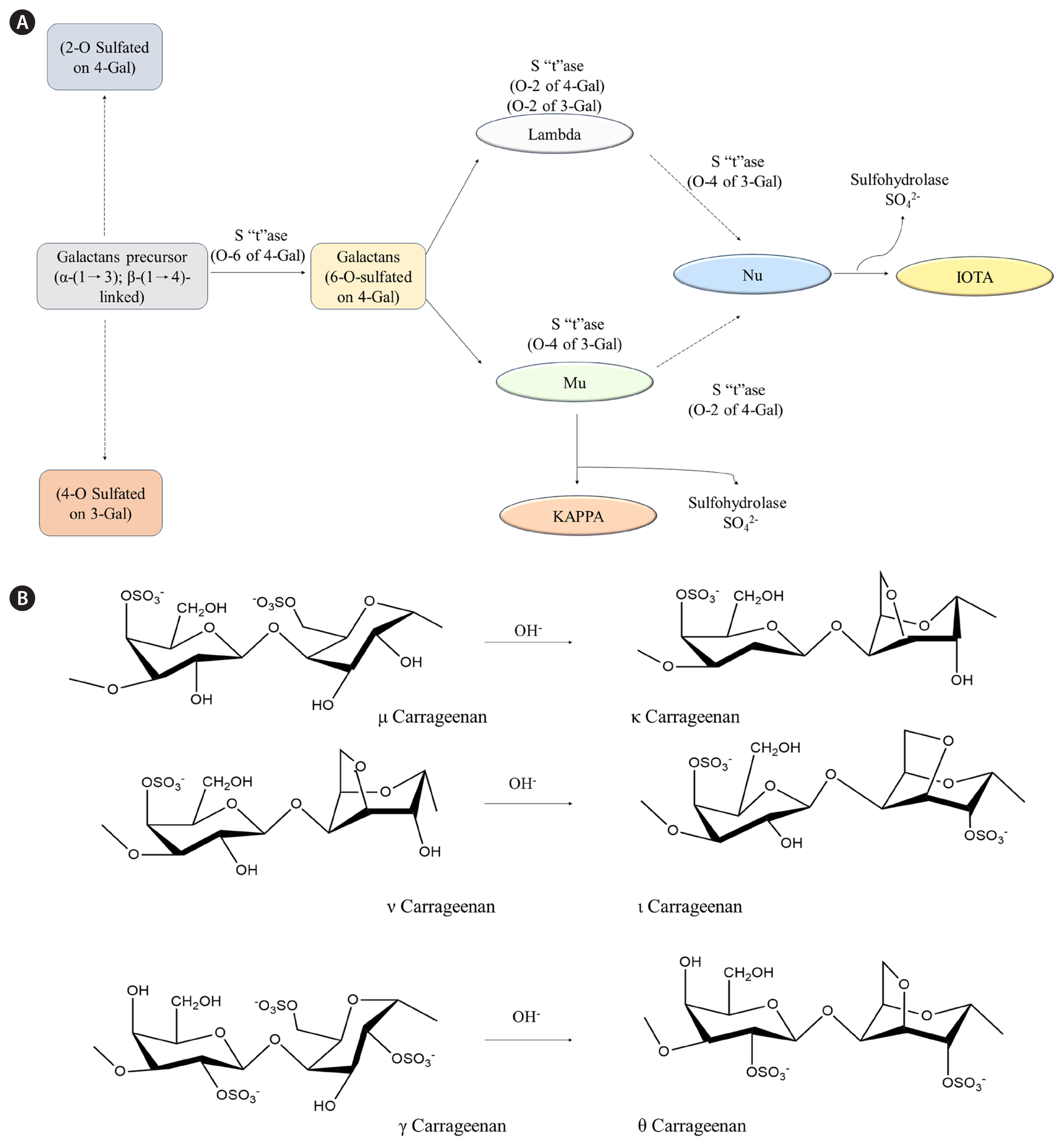
Fig. 4General structure and biosynthesis of ulvan. (A) Type A and B of ulvanobiuronic acid. (B) Type U of ulvanobioses. (C) The proposed mechanism of ulvan gel formation. 
Table 1Critical analysis of how the structural aspects influence biological activity Table 2Biological activities of sulfated polysaccharides and their structural features that affect commercial significance Table 3Challenges need to address for future developments in sulfated polysaccharides isolation from seaweeds in industrial scale REFERENCESAlboofetileh, M., Rezaei, M. & Tabarsa, M. 2019. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 31:1391–1402.
Ale, M. T., Mikkelsen, J. D. & Meyer, A. S. 2011. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs. 9:2106–2130.
Amorim, Rd. N. d. S., Rodrigues, J. A. G., Holanda, M. L., Quinderé, A. L. G., de Paula, R. C. M., Melo, V. M. M. & Benevides, N. M. B. 2012. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata
. Braz. Arch. Biol. Technol. 55:171–181.
Anisha, G. S., Augustianath, T., Padmakumari, S., Singhania, R. R., Pandey, A. & Patel, A. K. 2023. Ulvan from green macroalgae: bioactive properties advancing tissue engineering, drug delivery systems, food industry, agriculture and water treatment. Bioresour. Technol. Rep. 22:101457 pp.
Anisha, G. S., Padmakumari, S., Patel, A. K., Pandey, A. & Singhania, R. R. 2022. Fucoidan from marine macroalgae: biological actions and applications in regenerative medicine, drug delivery systems and food industry. Bioengineering. 9:472 pp.
Apostolova, E., Lukova, P., Baldzhieva, A., Katsarov, P., Nikolova, M., Iliev, I., Peychev, L., Trica, B., Oancea, F., Delattre, C. & Kokova, V. 2020. Immunomodulatory and anti-inflammatory effects of fucoidan: a review. Polymers. 12:2338 pp.
Barahona, T., Chandía, N. P., Encinas, M. V., Matsuhiro, B. & Zúñiga, E. A. 2011. Antioxidant capacity of sulfated polysaccharides from seaweeds: a kinetic approach. Food Hydrocoll. 25:529–535.
Bhardwaj, M., Padmavathy, T. K., Mani, S., Malarvizhi, R., Sali, V. K. & Vasanthi, H. R. 2020. Sulfated polysaccharide from Turbinaria ornata suppress lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages. Int. J. Biol. Macromol. 164:4299–4305.
Blakemore, W. R. & Harpell, A. R. 2009. Carrageenan. In : Imeson A., editor Food Stabilisers, Thickeners, and Gelling Agents. Blackwell Publishing Ltd, West Sussex, 73–94.
Campbell, R. & Hotchkiss, S. 2017. Carrageenan industry market overview. In : Hurtado A. Q., Critchley A. T., Neish I. C., editors Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce. Springer International Publishing, Cham, 193–205.
Chi, Y., Zhang, M., Wang, X., Fu, X., Guan, H. & Wang, P. 2020. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 157:75–82.
Choi, J.-I. & Kim, H.-J. 2013. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 97:358–362.
Ciancia, M., Quintana, I. & Cerezo, A. S. 2010. Overview of anticoagulant activity of sulfated polysaccharides from seaweeds in relation to their structures, focusing on those of green seaweeds. Curr. Med. Chem. 17:2503–2529.
Costa, L. S., Fidelis, G. P., Cordeiro, S. L., Oliveira, R. M., Sabry, D. A., Câmara, R. B. G., Nobre, L. T. D. B., Costa, M. S. S. P., Almeida-Lima, J., Farias, E. H. C., Leite, E. L. & Rocha, H. A. O. 2010. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 64:21–28.
da Silva, L. C. R. P., Todaro, V., do Carmo, F. A., Frattani, F. S., de Sousa, V. P., Rodrigues, C. R., Sathler, P. C. & Cabral, L. M. 2018. A promising oral fucoidan-based antithrombotic nanosystem: development, activity and safety. Nanotechnology. 29:165102 pp.
de Araújo, I. W. F., Rodrigues, J. A. G., Quinderé, A. L. G., de Fátima Teixeira Silva, J., de Freitas Maciel, G., Ribeiro, N. A., de Sousa Oliveira Vanderlei, E., Ribeiro, K. A., Chaves, H. V., Pereira, K. M. A., Bezerra, M. M. & Benevides, N. M. B. 2016. Analgesic and anti-inflammatory actions on bradykinin route of a polysulfated fraction from alga Ulva lactuca
. Int. J. Biol. Macromol. 92:820–830.
Delma, C., Ramalingam, K., Pandian, V., Baskar, A., Savarimuthu, I., Thangavelu, B. & Somasundaram, S. 2014. Abstract A4: antagonistic effects of sulphated polysaccharides from Turbinaria conoides (J. Agardh) on tumor cell migration and angiogenesis. Cancer Prev. Res. 1:A4 pp.
Fabrowska, J., Ibañez, E., Łęska, B. & Herrero, M. 2016. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 19:237–245.
Fernández-Díaz, C., Coste, O. & Malta, E.-J. 2017. Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immunostimulant effect in Solea senegalensis macrophages. Algal Res. 26:135–142.
Fitton, J. H., Stringer, D. N., Park, A. Y. & Karpiniec, S. S. 2019. Therapies from fucoidan: new developments. Mar Drugs. 17:571 pp.
Flórez-Fernández, N., Rodríguez-Coello, A., Latire, T., Bourgougnon, N., Torres, M. D., Buján, M., Muíños, A., Muiños, A., Meijide-Faílde, R., Blanco, F. J., Vaamonde-García, C. & Domínguez, H. 2023. Anti-inflammatory potential of ulvan. Int. J. Biol. Macromol. 253:126936 pp.
Gereniu, C. R. N., Saravana, P. S. & Chun, B.-S. 2018. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 196:309–317.
Hans, N., Malik, A. & Naik, S. 2021. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: mini review. Bioresour. Technol. Rep. 13:100623 pp.
Hayashi, K., Nakano, T., Hashimoto, M., Kanekiyo, K. & Hayashi, T. 2008. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 8:109–116.
Hotchkiss, S., Brooks, M., Campbell, R., Philp, K. & Trius, A. 2016. The use of carrageenan in food. In : Pereira L., editor Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects. Nova Science Publishers, New York, NY, 229–243.
Huang, L., Shen, M., Morris, G. A. & Xie, J. 2019. Sulfated polysaccharides: immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 92:1–11.
Hwang, E. K., Boo, G. H., Graf, L., Yarish, C., Yoon, H. S. & Kim, J. K. 2022. Kelps in Korea: from population structure to aquaculture to potential carbon sequestratio. Algae. 37:85–103.
Ibrahim, M. I. A., Amer, M. S., Ibrahim, H. A. H. & Zaghloul, E. H. 2022. Considerable production of ulvan from Ulva lactuca with special emphasis on its antimicrobial and anti-fouling properties. Appl. Biochem. Biotechnol. 194:3097–3118.
Imeson, A. 2009. Carrageenan and furcellaran. In : Phillips G. O., Williams P. A., editors Handbook of Hydrocolloids. 2nd ed. Woodhead Publishing, Cambridge, 164–185.
Jaulneau, V., Lafitte, C., Jacquet, C., Fournier, S., Salamagne, S., Briand, X., Esquerré-Tugayé, M.-T. & Dumas, B. 2010. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic scid signaling pathway. J. Biotechnol. 2010. 525291.
Jayasinghe, P. S., Pahalawattaarachchi, V. & Ranaweera, K. K. D. S. 2016. Effect of extraction methods on the yield and physiochemical properties of polysaccharides extracted from seaweed available in Sri Lanka. Poult. Fish. Wildl. Sci. 4:1 pp.
Jayawardena, T. U., Nagahawatta, D. P., Fernando, I. P. S., Kim, Y.-T., Kim, J.-S., Kim, W.-S., Lee, J. S. & Jeon, Y.-J. 2022. A review on fucoidan structure, extraction techniques, and its role as an immunomodulatory agent. Mar. Drugs . 20:755 pp.
Jayawardena, T. U., Sanjeewa, K. K. A., Lee, H.-G., Nagahawatta, D. P., Yang, H.-W., Kang, M.-C. & Jeon, Y.-J. 2020a. Particulate matter-induced inflammation/oxidative stress in macrophages: fucosterol from Padina boryana as a potent protector, activated via NF-κB/MAPK pathways and Nrf2/HO-1 involvement. Mar. Drugs. 18:628 pp.
Jayawardena, T. U., Sanjeewa, K. K. A., Nagahawatta, D. P., Lee, H.-G., Lu, Y.-A., Vaas, A. P. J. P., Abeytunga, D. T. U., Nanayakkara, C. M., Lee, D.-S. & Jeon, Y.-J. 2020b. Anti-inflammatory effects of sulfated polysaccharide from Sargassum swartzii in macrophages via blocking TLR/NF-Κb signal transduction. Mar. Drugs. 18:601 pp.
Jayawardhana, H. H. A. C. K., Lee, H.-G., Liyanage, N. M., Nagahawatta, D. P., Ryu, B. & Jeon, Y.-J. 2023. Structural characterization and anti-inflammatory potential of sulfated polysaccharides from Scytosiphon lomentaria: attenuate inflammatory signaling pathways. J. Funct. Foods. 102:105446 pp.
Jiksing, C., Ongkudon, M. M., Thien, V. Y., Rodrigues, K. F. & Yong, WT L. 2022. Recent advances in seaweed seedling production: a review of eucheumatoids and other valuable seaweeds. Algae. 37:105–121.
Karnjanapratum, S. & You, S. 2011. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 48:311–318.
Kidgell, J. T., Glasson, C. R. K., Magnusson, M., Vamvounis, G., Sims, I. M., Carnachan, S. M., Hinkley, S. F. R., Lopata, A. L., de Nys, R. & Taki, A. C. 2020. The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. Int. J. Biol. Macromol. 150:839–848.
Kikionis, S., Koromvoki, M., Tagka, A., Polichronaki, E., Stratigos, A., Panagiotopoulos, A., Kyritsi, A., Karalis, V., Vitsos, A., Rallis, M., Ioannou, E. & Roussis, V. 2022. Ulvan-based nanofibrous patches enhance wound healing of skin trauma resulting from cryosurgical treatment of keloids. Mar Drugs. 20:551 pp.
Knoop, J., Barrento, S., Lewis, R., Walter, B. & Griffin, J. N. 2022. Incorporating concepts of biodiversity into modern aquaculture: macroalgal species richness enhances bioremediation efficiency in a lumpfish hatchery. Algae. 37:213–226.
Lahaye, M. & Robic, A. 2007. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 8:1765–1774.
Lee, H.-G., Nagahawatta, D. P., Liyanage, N. M., Jayawardhana, H. H. A. C. K., Yang, F., Je, J.-G., Kang, M.-C., Kim, H.-S. & Jeon, Y.-J. 2022. Structural characterization and anti-inflammatory activity of fucoidan isolated from Ecklonia maxima stipe. Algae. 37:239–247.
Lee, J.-B., Yamagaki, T., Maeda, M. & Nakanishi, H. 1998. Rhamnan sulfate from cell walls of Monostroma latissimum
. Phytochemistry. 48:921–925.
Li, Q., Wang, X., Wan, Y., Hu, X., Liu, J. & Wang, J. 2023.
In vivo immunomodulatory activity offucoidan from brown alga Undaria pinnatifida in sarcoma 180-bearing mice. J. Funct. Foods. 103:105486 pp.
Li, R., Zhou, Q.-L., Chen, S.-T., Tai, M.-R., Cai, H.-Y., Ding, R., Liu, X.-F., Chen, J.-P., Luo, L.-X. & Zhong, S.-Y. 2022. Chemical characterization and immunomodulatory activity of fucoidan from Sargassum hemiphyllum
. Mar Drugs. 21:18 pp.
Li, W., Jiang, N., Li, B., Wan, M., Chang, X., Liu, H., Zhang, L., Yin, S., Qi, H. & Liu, S. 2018. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 113:971–975.
Lin, Y., Qi, X., Liu, H., Xue, K., Xu, S. & Tian, Z. 2020. The anti-cancer effects of fucoidan: a review of both in vivo and in vitro investigations. Cancer Cell Int. 20:154 pp.
Little, S. M., Senhorinho, G. N. A., Saleh, M., Basiliko, N. & Scott, J. A. 2021. Antibacterial compounds in green microalgae from extreme environments: a review. Algae. 36:61–72.
Liyanage, N. M., Nagahawatta, D. P., Jayawardena, T. U. & Jeon, Y.-J. 2023a. The role of seaweed polysaccharides in gastrointestinal health: protective effect against inflammatory bowel disease. Life. 13:1026 pp.
Liyanage, N. M., Nagahawatta, D. P., Jayawardena, T. U., Sanjeewa, K. K. A., Jayawrdhana, H. H. A. C. K., Kim, J.-I. & Jeon, Y.-J. 2023b. Sulfated polysaccharides from seaweeds: a promising strategy for combatting viral diseases. A review. Mar. Drugs. 21:461 pp.
Lüning, K. & Pang, S. 2003. Mass cultivation of seaweeds: current aspects and approaches. J. Appl. Phycol. 15:115–119.
Lu, W., Yang, Z., Chen, J., Wang, D. & Zhang, Y. 2021. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 272:118526 pp.
Manikandan, R., Parimalanandhini, D., Mahalakshmi, K., Beulaja, M., Arumugam, M., Janarthanan, S., Palanisamy, S., You, S. & Prabhu, N. M. 2020. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 160:1263–1276.
Maray, S. O., Abdel-Kareem, M. S. M., Mabrouk, M. E. M., El-Halmouch, Y. & Makhlof, M. E. M. 2023.
In vitro assessment of antiviral, antimicrobial, antioxidant and anticancer activities of ulvan extracted from the green seaweed Ulva lactuca
. Thalassas Int. J. Mar. Sci. 39:779–790.
Mauray, S., Sternberg, C., Theveniaux, J., Millet, J., Sinquin, C., Tapon-Bretaudière, J. & Fischer, A. M. 1995. Venous antithrombotic and anticoagulant activities of a fucoidan fraction. Thromb. Haemost. 74:1280–1285.
Moawad, M. N., El-Sayed, A. A. M., Abd El Latif, H. H., El-Naggar, N. A., El-Din, N. G. S. & Tadros, H. R. Z. 2022. Chemical characterization and biochemical activity of polysaccharides isolated from Egyptian Ulva fasciata Delile. Oceanologia. 64:117–130.
Morán-Santibañez, K., Cruz-Suárez, L. E., Ricque-Marie, D., Robledo, D., Freile-Pelegrín, Y., Peña-Hernández, M. A., Rodríguez-Padilla, C. & Trejo-Avila, L. M. 2016. Synergistic effects of sulfated polysaccharides from Mexican seaweeds against measles virus. Biomed Res. Int. 2016. 8502123.
Muthukumar, J., Chidambaram, R. & Sukumaran, S. 2021. Sulfated polysaccharides and its commercial applications in food industries: a review. J. Food Sci. Technol. 58:2453–2466.
Nagahawatta, D., Sanjeewa, K. K. A., Jayawardena, T. U., Kim, H.-S., Yang, H.-W., Jiang, Y., Je, J.-G., Lee, T.-K. & Jeon, Y.-J. 2021. Drying seaweeds using hybrid hot water Goodle dryer (HHGD): comparison with freeze-dryer in chemical composition and antioxidant activity. Fish Aquat Sci. 24:19–31.
Nagahawatta, D. P., Liyanage, N. M., Jayawardhana, H. H. A. C. K., Lee, H.-G., Jayawardena, T. U. & Jeon, Y.-J. 2022. Anti-fine dust effect of fucoidan extracted from Ecklonia maxima leaves in macrophages via inhibiting inflammatory signaling pathways. Mar. Drugs. 20:413 pp.
Ortega-Barria, E. & Boothroyd, J. C. 1999. A toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 274:1267–1276.
Palanisamy, S., Vinosha, M., Marudhupandi, T., Rajasekar, P. & Prabhu, N. M. 2017. Isolation of fucoidan from Sargassum polycystum brown algae: structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 102:405–412.
Park, H. Y., Han, M. H., Park, C., Jin, C.-Y., Kim, G.-Y., Choi, I.-W., Kim, N. D., Nam, T.-J., Kwon, T. K. & Choi, Y. H. 2011. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 49:1745–1752.
Piriz, M. L., Eyras, M. C. & Rostagno, C. M. 2003. Changes in biomass and botanical composition of beach-cast seaweeds in a disturbed coastal area from Argentine Patagonia. J. Appl. Phycol. 15:67–74.
Pradhan, B., Patra, S., Nayak, R., Behera, C., Dash, S. R., Nayak, S., Sahu, B. B., Bhutia, S. K. & Jena, M. 2020. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: a journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 164:4263–4278.
Rodriguez-Jasso, R. M., Mussatto, S. I., Pastrana, L., Aguilar, C. N. & Teixeira, J. A. 2011. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 86:1137–1144.
Salehi, B., Sharifi-Rad, J., Seca, A. M. L., Pinto, D. C. G. A., Michalak, I., Trincone, A., Mishra, A. P., Nigam, M., Zam, W. & Martins, N. 2019. Current trends on seaweeds: looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 24:4182 pp.
Sánchez, R. A. R., Matulewicz, M. C. & Ciancia, M. 2022. NMR spectroscopy for structural elucidation of sulfated polysaccharides from red seaweeds. Int. J. Biol. Macromol. 199:386–400.
Saravana, P. S., Cho, Y.-N., Patil, M. P., Cho, Y.-J., Kim, G.-D., Park, Y. B., Woo, H.-C. & Chun, B.-S. 2018. Hydrothermal degradation of seaweed polysaccharide: characterization and biological activities. Food Chem. 268:179–187.
Shao, P., Chen, M., Pei, Y. & Sun, P. 2013. In intro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata
. Int. J. Biol. Macromol. 59:295–300.
Shen, P., Yin, Z., Qu, G. & Wang, C. 2018. Fucoidan and its health benefits. In : Qin Y., editor Bioactive Seaweeds for Food Applications. Academic Press, London, 223–238.
Snethlage, J. S., de Koning, S., Giesbers, E., Veraart, J. A., Debrot, A. O., Harkes, I., van den Burg, S. W. K. & Hamon, K. G. 2023. Knowledge needs in realising the full potential of seaweed for world food provisioning. Glob. Food Sec. 37:100692 pp.
Song, Y., He, P., Rodrigues, A. L., Datta, P., Tandon, R., Bates, J. T., Bierdeman, M. A., Chen, C., Dordick, J., Zhang, F. & Linhardt, R. J. 2021. Anti-SARS-CoV-2 activity of rhamnan sulfate from Monostroma nitidum
. Mar Drugs. 19:685 pp.
Therkelsen, G. H. 1993. Carrageenan. In : Whistler R. L., Bemiller J. N., editors Industrial Gums. 3rd ed. Academic Press, London, 145–180.
Tran, T. T. V., Truong, H. B., Tran, N. H. V., Quach, T. M. T., Nguyen, T. N., Bui, M. L., Yuguchi, Y. & Thanh, T. T. T. 2018. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata
. Nat. Prod. Res. 32:2291–2296.
Tziveleka, L.-A., Pippa, N., Ioannou, E., Demetzos, C. & Roussis, V. 2022. Development of ulvan-containing liposomes as antibacterial drug delivery platforms. J. Funct. Biomater. 13:186 pp.
Venkatesan, J., Singh, S. K., Anil, S., Kim, S.-K. & Shim, M. S. 2018. Preparation, characterization and biological applications of biosynthesized silver nanoparticles with chitosan-fucoidan coating. Molecules. 23:1429 pp.
Wang, J., Zhang, Q., Zhang, Z. & Li, Z. 2008. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica
. Int. J. Biol. Macromol. 42:127–132.
Wang, L., Oh, J.-Y., Kim, Y.-S., Lee, H.-G., Lee, J.-S. & Jeon, Y.-J. 2020. Anti-photoaging and anti-melanogenesis effects of fucoidan isolated from Hizikia fusiforme and its underlying mechanisms. Mar Drugs. 18:427 pp.
Wei, Q., Fu, G., Wang, K., Yang, Q., Zhao, J., Wang, Y., Ji, K. & Song, S. 2022. Advances in research on antiviral activities of sulfated polysaccharides from seaweeds. Pharmaceuticals. 15:581 pp.
Weiner, M. L. 2014. Food additive carrageenan: part II: a critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 44:244–269.
Wiencke, C. & Bischof, K. 2012. Seaweed biology. 219:Springer, Heidelberg, 507 pp.
Wijesinghe, W. A. J. P. & Jeon, Y.-J. 2012. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Carbohydr. Polym. 88:13–20.
Wu, G.-J., Shiu, S.-M., Hsieh, M.-C. & Tsai, G.-J. 2016. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium
. Food Hydrocoll. 53:16–23.
Yang, M. Y. & Kim, M. S. 2022. Phylogeography of the economic seaweeds Chondrus (Gigartinales, Rhodophyta) in the northwest Pacific based on rbcL and COI-5P genes. Algae. 37:135–147.
Yoo, H. J., You, D.-J. & Lee, K.-W. 2019. Characterization and immunomodulatory effects of high molecular weight fucoidan fraction from the sporophyll of Undaria pinnatifida in cyclophosphamide-induced immunosuppressed mice. Mar Drugs. 17:447 pp.
Zayed, A., Avila-Peltroche, J., El-Aasr, M. & Ulber, R. 2022. Sulfated galactofucans: an outstanding class of fucoidans with promising bioactivities. Mar Drugs. 20:412 pp.
Zhang, L., Wang, X., Hua, Q., Wang, J., Liu, J. & Yang, Y. 2020. Synthesis and immunomodulatory activity of the sulfated tetrasaccharide motif of type B ulvanobiuronic acid 3-sulfate. Org. Biomol. Chem. 18:7932–7935.
|
|
||||||||||||||||||||||||||||||||||||||